Question: Answer d) and e) only [i know how to do a b c] Q1. One mole of n-butane is burned with an excess amount of
Answer d) and e) only [i know how to do a b c]
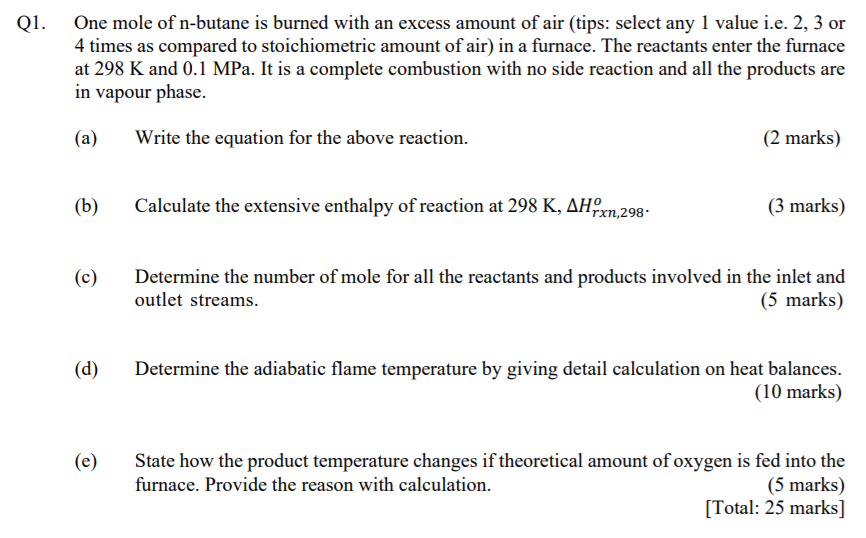
Q1. One mole of n-butane is burned with an excess amount of air (tips: select any 1 value i.e. 2, 3 or 4 times as compared to stoichiometric amount of air) in a furnace. The reactants enter the furnace at 298 K and 0.1 MPa. It is a complete combustion with no side reaction and all the products are in vapour phase. (a) Write the equation for the above reaction. (2 marks) (6) Calculate the extensive enthalpy of reaction at 298 K, AHOX rxn,298 (3 marks) (c) Determine the number of mole for all the reactants and products involved in the inlet and outlet streams. 5 (5 marks) (d) Determine the adiabatic flame temperature by giving detail calculation on heat balances. (10 marks) (e) State how the product temperature changes if theoretical amount of oxygen is fed into the furnace. Provide the reason with calculation. (5 marks) [Total: 25 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


