Question: answer EACH PART very clearly please dont make it confusing , this is chem 2 6 0 0 , physical chemistry course ! ! !
answer EACH PART very clearly please dont make it confusingthis is chem
physical chemistry course
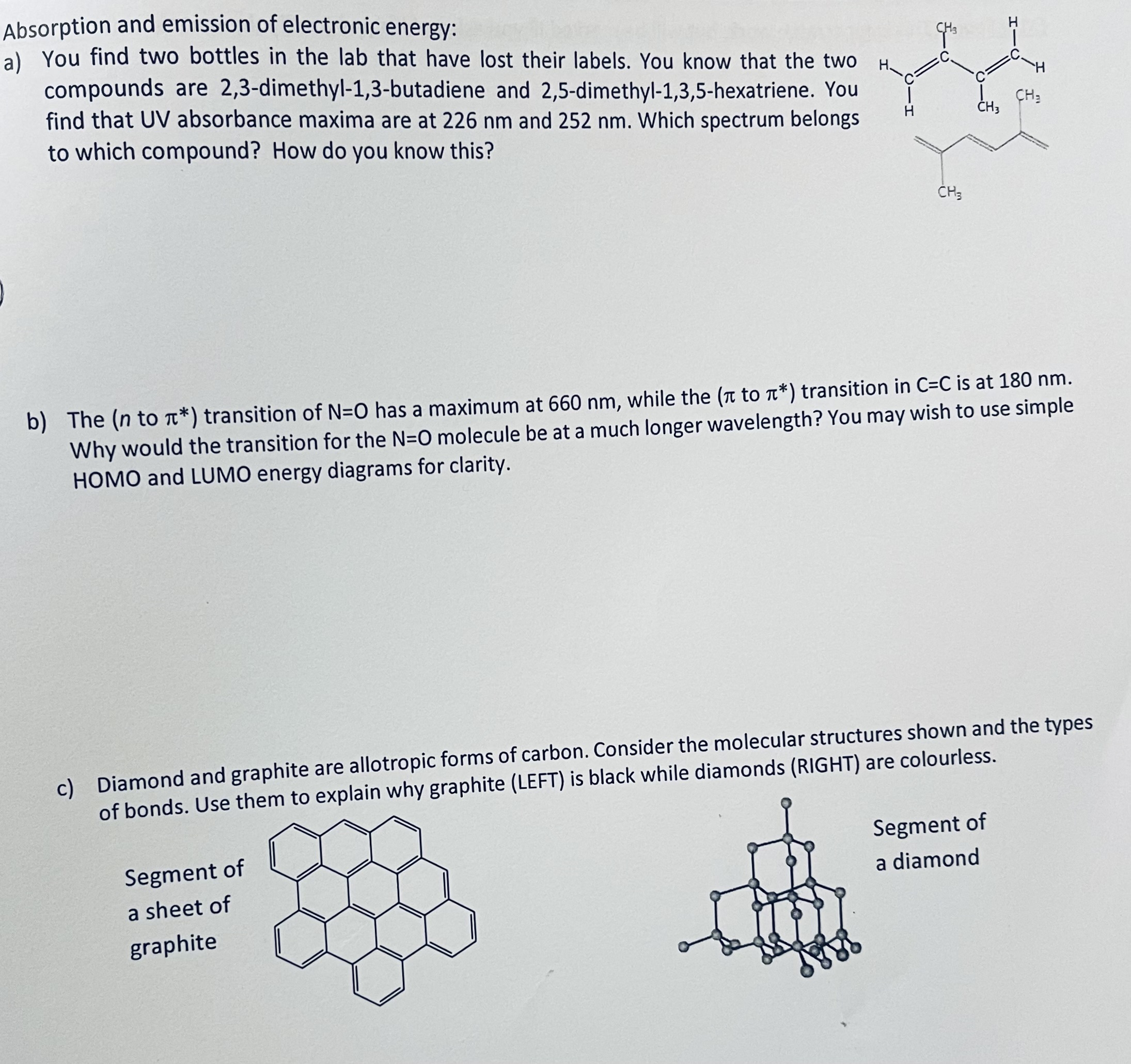
Absorption and emission of electronic energy:
a You find two bottles in the lab that have lost their labels. You know that the two
compounds are dimethylbutadiene and dimethylhexatriene. You
find that UV absorbance maxima are at and Which spectrum belongs
to which compound? How do you know this?
b The to transition of has a maximum at while the to transition in is at
Why would the transition for the molecule be at a much longer wavelength? You may wish to use simple
HOMO and LUMO energy diagrams for clarity.
c Diamond and graphite are allotropic forms of carbon. Consider the molecular structures shown and the types
of bonds. Use them to explain why graphite LEFT is black while diamonds RIGHT are colourless.
Segment of
a sheet of
graphite
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


