Question: Which of the tollowing statements is correct? (A) If 0.1M CH3COOH aqueous solution (K. = 1.8 x 10-5) is diluted at 25C, then [H*]
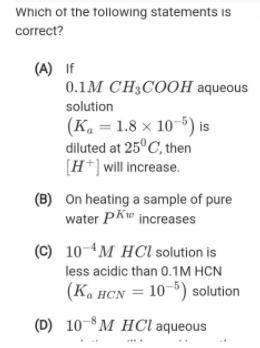
Which of the tollowing statements is correct? (A) If 0.1M CH3COOH aqueous solution (K. = 1.8 x 10-5) is diluted at 25C, then [H*] will increase. (B) On heating a sample of pure water Pkw increases (C) 10-4M HCl solution is less acidic than 0.1M HCN (Ka HCN = 10-5) solution (D) 10-8M HCl aqueous
Step by Step Solution
3.36 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


