Question: Answer for a, b, and c (7%) Problem 11: The figure shows the electron energy-level diagram during a collision between a helium atom and a
Answer for a, b, and c
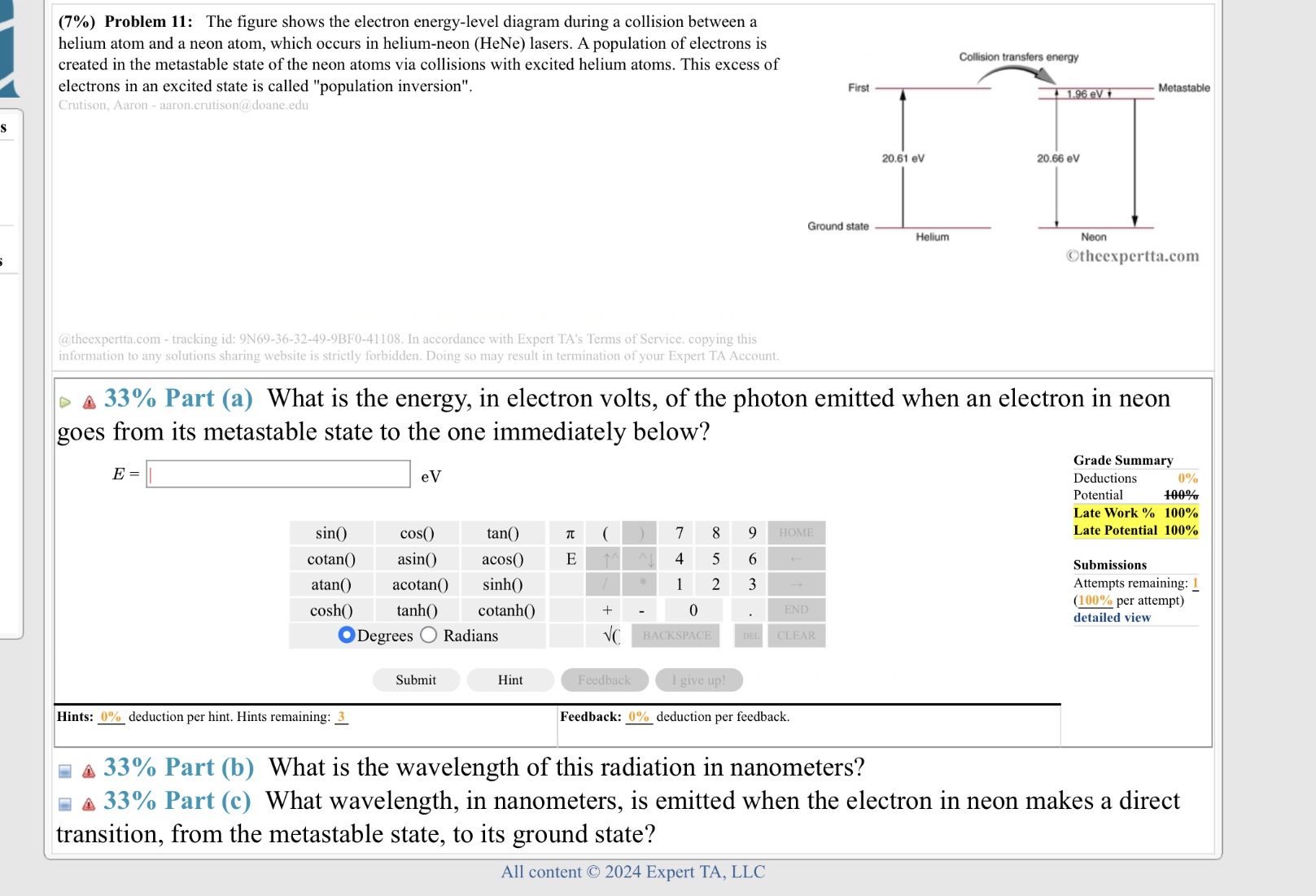
(7%) Problem 11: The figure shows the electron energy-level diagram during a collision between a helium atom and a neon atom, which occurs in helium-neon (HeNe) lasers. A population of electrons is created in the metastable state of the neon atoms via collisions with excited helium atoms. This excess of Collision transfers energy electrons in an excited state is called "population inversion". First 1.96 ev Metastable Crutison, Aaron - aaron.crutison@doane.edu 20.61 ev 20.66 ev Ground state Helium Neon Otheexpertta.com @theexpertta.com - tracking id: 9N69-36-32-49-9BF0-41108. In accordance with Expert TA's Terms of Service. copying this information to any solutions sharing website is strictly forbidden. Doing so may result in termination of your Expert TA Account. A 33% Part (a) What is the energy, in electron volts, of the photon emitted when an electron in neon goes from its metastable state to the one immediately below? Grade Summary E = e V Deductions 0% Potential 100% Late Work % 100% sino cos() tan( 7t 8 9 HOME Late Potential 100% cotan() asin( acos() E 5 6 Submissions atan() acotan() sinh() 3 Attempts remaining: 1 (100% per attempt) cosh() tanh() cotanh() END detailed view ODegrees O Radians VC BACKSPACE CLEAR Submit Hint Feedback I give up! Hints: 0% deduction per hint. Hints remaining: 3 Feedback: 0% deduction per feedback. 4 33% Part (b) What is the wavelength of this radiation in nanometers? 4 33% Part (c) What wavelength, in nanometers, is emitted when the electron in neon makes a direct transition, from the metastable state, to its ground state? All content 2024 Expert TA, LLC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


