Question: answer number 5 please 5. Ethane, C2H6, dissociates into methyl radicals at 700.C with a rate constant, k=5.5104 s1. Determine the rate constant at 800.C,

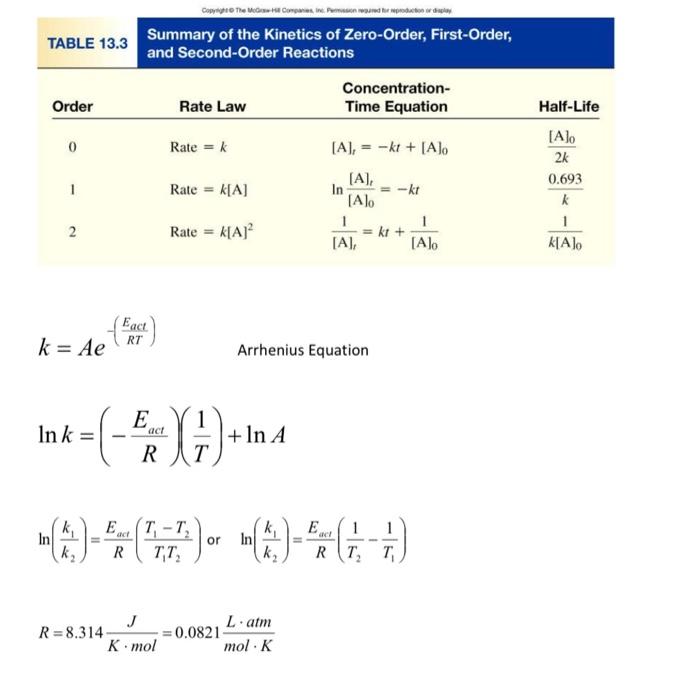
5. Ethane, C2H6, dissociates into methyl radicals at 700.C with a rate constant, k=5.5104 s1. Determine the rate constant at 800.C, given the activation energy of the reaction is k=Ae(RTEact) Arrhenius Equation lnk=(REact)(T1)+lnA ln(k2k1)=REact(T1T2T1T2) or ln(k2k1)=REact(T21T11) R=8.314KmolJ=0.0821molKLatm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


