Question: Answer only part b Please pick it up only if you are going to answer thoroughly. Thank you. Q4 (a) Blending hydrogen with natural gas
Answer only part b
Please pick it up only if you are going to answer thoroughly. Thank you.
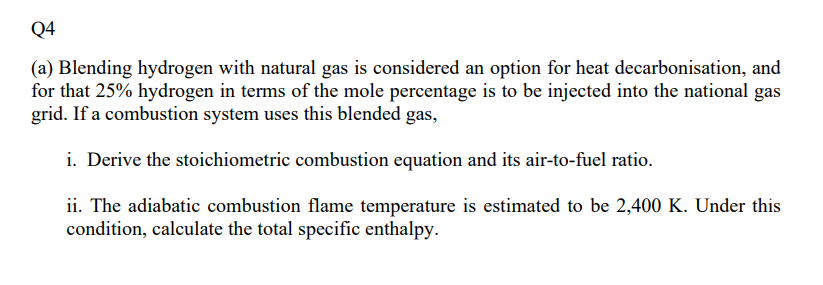
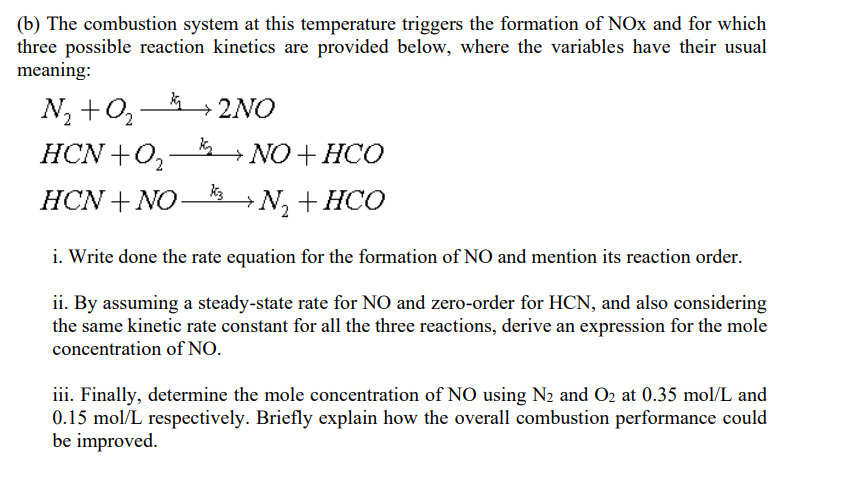
Q4 (a) Blending hydrogen with natural gas is considered an option for heat decarbonisation, and for that 25% hydrogen in terms of the mole percentage is to be injected into the national gas grid. If a combustion system uses this blended gas, i. Derive the stoichiometric combustion equation and its air-to-fuel ratio. ii. The adiabatic combustion flame temperature is estimated to be 2,400 K. Under this condition, calculate the total specific enthalpy. (b) The combustion system at this temperature triggers the formation of NOx and for which three possible reaction kinetics are provided below, where the variables have their usual meaning: N2 +02_2NO HCN+), k + NO + HCO ki HCN + NO -N,+HCO i. Write done the rate equation for the formation of NO and mention its reaction order. ii. By assuming a steady-state rate for NO and zero-order for HCN, and also considering the same kinetic rate constant for all the three reactions, derive an expression for the mole concentration of NO. iii. Finally, determine the mole concentration of NO using N2 and O2 at 0.35 mol/L and 0.15 mol/L respectively. Briefly explain how the overall combustion performance could be improved
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


