Question: answer only please. thank you Question 33 1 pts Given the following thermochemical equation: CaCO3(s) CaO(s) +CO2 (g) AH=177.8 kJ/mol What is the change in
answer only please. thank you
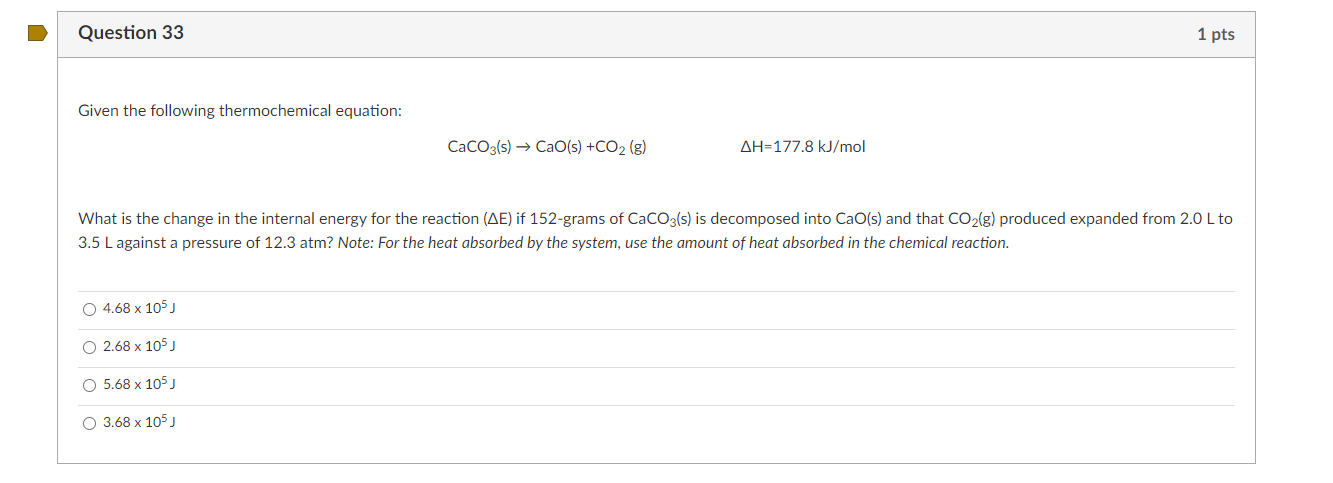
Question 33 1 pts Given the following thermochemical equation: CaCO3(s) CaO(s) +CO2 (g) AH=177.8 kJ/mol What is the change in the internal energy for the reaction (AE) if 152-grams of CaCO3(s) is decomposed into CaO(s) and that CO2(g) produced expanded from 2.0 L to 3.5 L against a pressure of 12.3 atm? Note: For the heat absorbed by the system, use the amount of heat absorbed in the chemical reaction. O 4.68 x 105) 2.68 x 105) O 5.68 x 105) O 3.68 x 105)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


