Question: answer options for question 1: solid, liquid, gas options for question 2 & 3: nothing, it will melt, it will freeze, it will boil, it
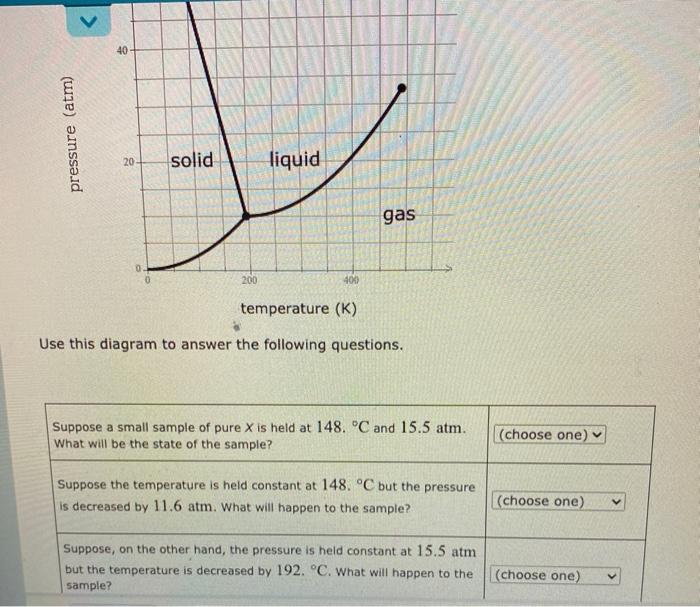
40 pressure (atm) 20 solid liquid gas 0 200 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 148. C and 15.5 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at 148. C but the pressure is decreased by 11.6 atm. What will happen to the sample? (choose one) Suppose, on the other hand, the pressure is held constant at 15.5 atm but the temperature is decreased by 192. C. What will happen to the sample? (choose one)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


