Question: answer part d please This question has multiple parts. Work all the parts to get the me Data in the table were collected at 540K
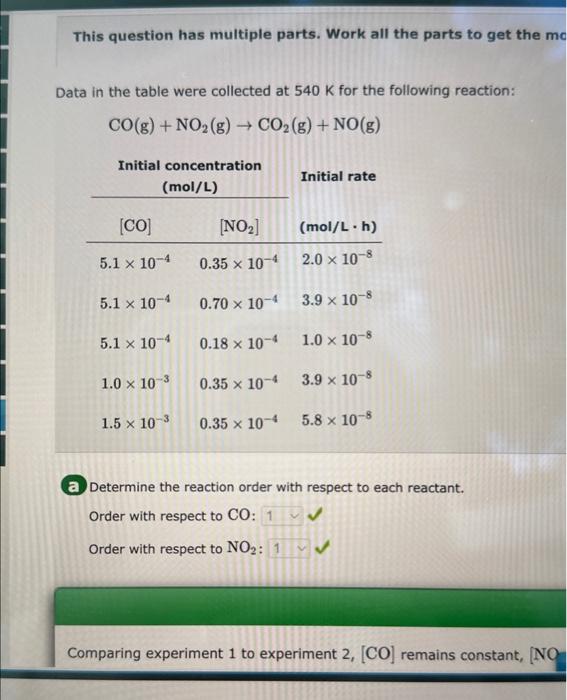
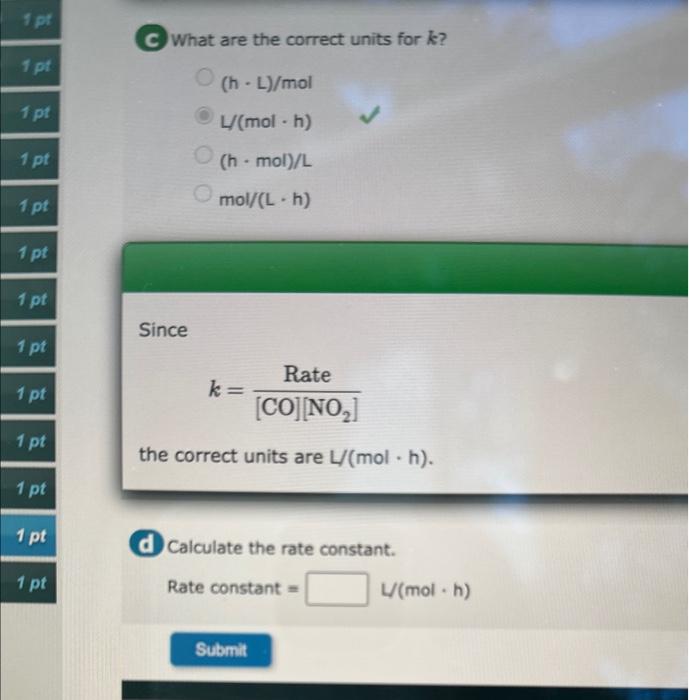
This question has multiple parts. Work all the parts to get the me Data in the table were collected at 540K for the following reaction: CO(g)+NO2(g)CO2(g)+NO(g) a Determine the reaction order with respect to each reactant. Order with respect to CO : Order with respect to NO2 : What are the correct units for k ? (hL)/molL(molh)(hmol)/Lmol/(Lh) Since k=[CO][NO2]Rate the correct units are L/(molh). Calculate the rate constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


