Question: answer please :( example of a typical complex buffered solution complex solutions from stocks. Below is an enriched mitochondria prepared from yeast cells. we will
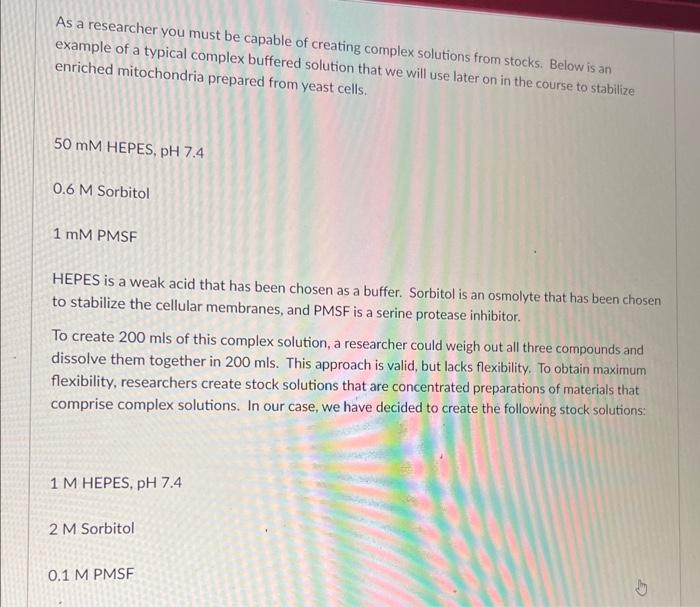
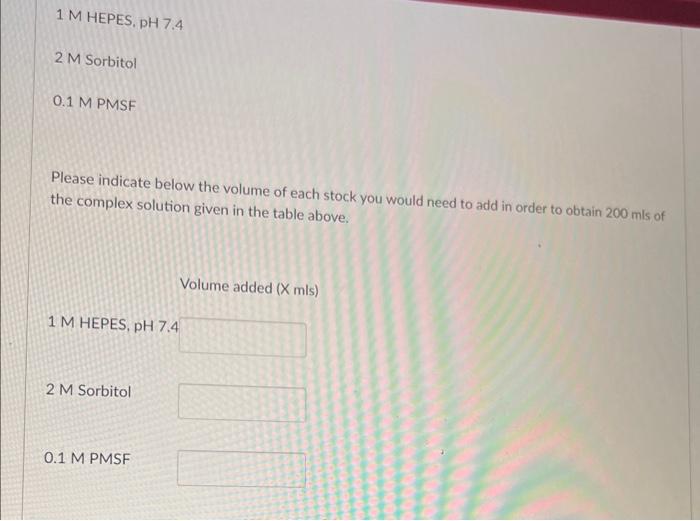
example of a typical complex buffered solution complex solutions from stocks. Below is an enriched mitochondria prepared from yeast cells. we will use later on in the course to stabilize 50 mM HEPES, pH 7.4 0.6 M Sorbitol 1 mM PMSF HEPES is a weak acid that has been chosen as a buffer. Sorbitol is an osmolyte that has been chosen to stabilize the cellular membranes, and PMSF is a serine protease inhibitor. To create 200mls of this complex solution, a researcher could weigh out all three compounds and dissolve them together in 200mls. This approach is valid, but lacks flexibility. To obtain maximum flexibility, researchers create stock solutions that are concentrated preparations of materials that comprise complex solutions. In our case, we have decided to create the following stock solutions: 1M HEPES, pH7.4 2 M Sorbitol 0.1MPMSF Please indicate below the volume of each stock you would need to add in order to obtain 200mls of the complex solution given in the table above. Volume added (Xmls) 1M HEPES, pH7.4 2 M Sorbitol 0.1 MPMSF
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


