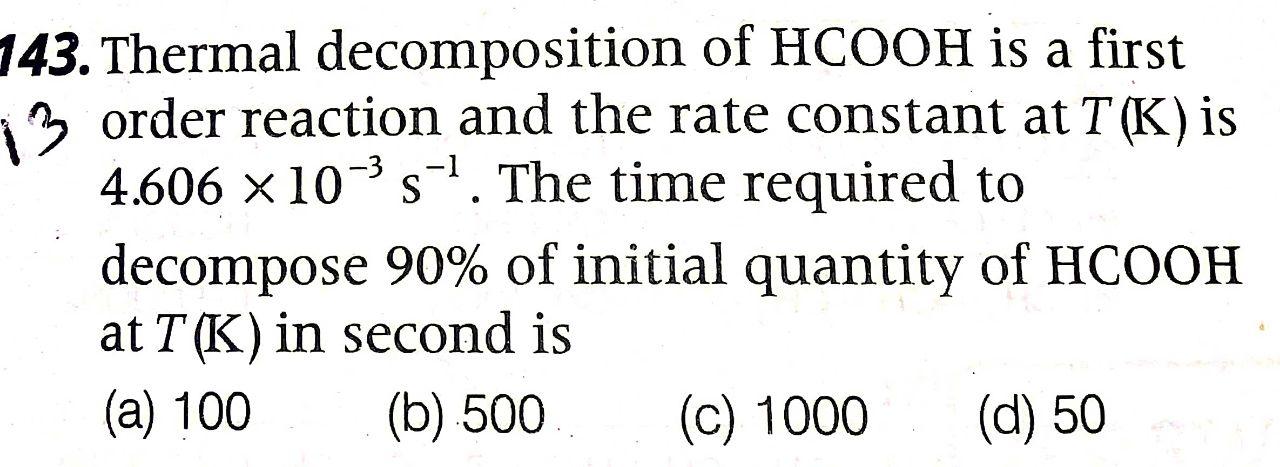
Question: Answer plz with correct option -1 S 143. Thermal decomposition of HCOOH is a first 13 order reaction and the rate constant at T (K)
 Answer plz with correct option
Answer plz with correct option
-1 S 143. Thermal decomposition of HCOOH is a first 13 order reaction and the rate constant at T (K) is 4.606 10-'5-7. The time required to decompose 90% of initial quantity of HCOOH at T (K) in second is (a) 100 (b) 500 (C) 1000 (d) 50
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


