Question: Answer questions a- C about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest
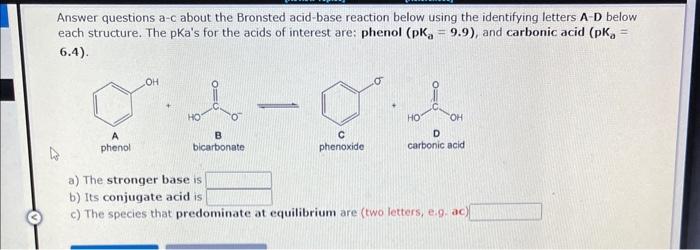
Answer questions a- C about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: phenol (pKa=9.9), and carbonic acid (pKa= 6.4). phenol bicarbonate phenoxide carbonic acid a) The stronger base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.9.ac)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


