Question: Answer should be well explained. = Methane (CH4) is burned with air (79% N2 and 21% 02 by volume) at atmospheric pressure. The molar analysis
Answer should be well explained.
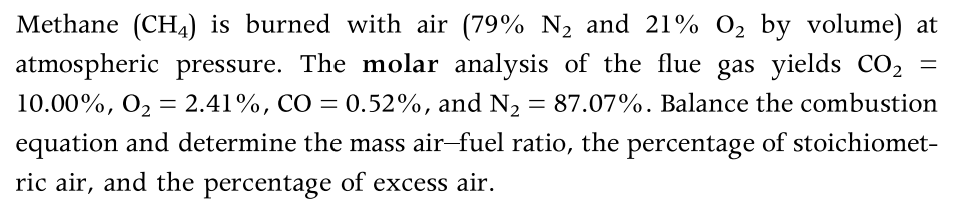
= Methane (CH4) is burned with air (79% N2 and 21% 02 by volume) at atmospheric pressure. The molar analysis of the flue gas yields CO2 10.00%, 02 = 2.41%, CO = 0.52%, and N2 = 87.07%. Balance the combustion equation and determine the mass air-fuel ratio, the percentage of stoichiomet- ric air, and the percentage of excess air. = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


