Question: Answer the following questions below 1.There are two groups (freshmen and juniors), and you are interested in whether freshmen express larger preference for social media
Answer the following questions below 1.There are two groups (freshmen and juniors), and you are interested in whether freshmen express larger preference for social media than juniors do:
1) Formulate a (alternative) hypothesis
2) What are H0 and H1
3) What is the proper type of test for hypothesis test?
4) Does it need one-tail test or two-tail test?
Q7. There is a group (females), and you are interested in whether their preference for iPhone is larger than that for Galaxy;
1) Formulate a (alternative) hypothesis
2) What are H0 and H1
3) What is the proper type of test for hypothesis test?
4) Does it need one-tail test or two-tail test?
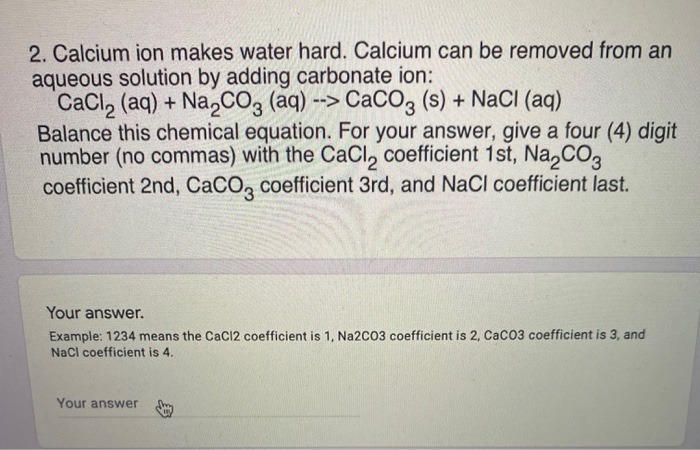
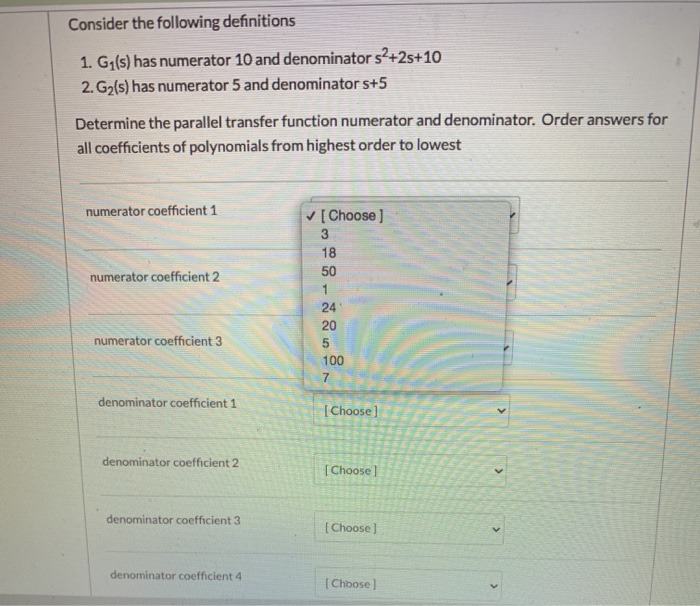
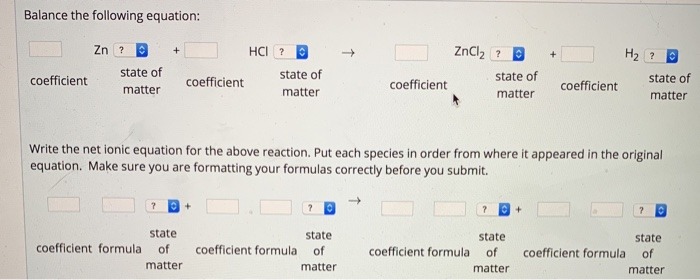
2. Calcium ion makes water hard. Calcium can be removed from an aqueous solution by adding carbonate ion: CaCl2 (aq) + Na2COg (aq) --> CaCO? (s) + NaCl (aq) Balance this chemical equation. For your answer, give a four (4) digit number (no commas) with the CaCl, coefficient 1st, Na,CO, coefficient 2nd, CaCO, coefficient 3rd, and NaCl coefficient last. Your answer. Example: 1234 means the CaCl2 coefficient is 1, Na2CO3 coefficient is 2, CaCO3 coefficient is 3, and NaCl coefficient is 4. Your answerConsider the following definitions 1. G1(s) has numerator 10 and denominator s2+2s+10 2. G2(s) has numerator 5 and denominator s+5 Determine the parallel transfer function numerator and denominator. Order answers for all coefficients of polynomials from highest order to lowest numerator coefficient 1 [ Choose ] 3 18 numerator coefficient 2 50 1 24 20 numerator coefficient 3 5 100 7 denominator coefficient 1 [ Choose ] denominator coefficient 2 [ Choose ] denominator coefficient 3 [ Choose ] denominator coefficient 4 [ Choose ]Balance the following equation: Zn ? HCI ? ZnClz ? ? state of coefficient state of coefficient state of coefficient state of matter matter coefficient matter matter Write the net ionic equation for the above reaction. Put each species in order from where it appeared in the original equation. Make sure you are formatting your formulas correctly before you submit. state state state state coefficient formula of coefficient formula of coefficient formula of coefficient formula of matter matter matter matter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


