Question: Answer the given question using the Gibbs tree energy vs. composition change diagram of the G.Fe binary solution at 1355C. The cliagram is given below.
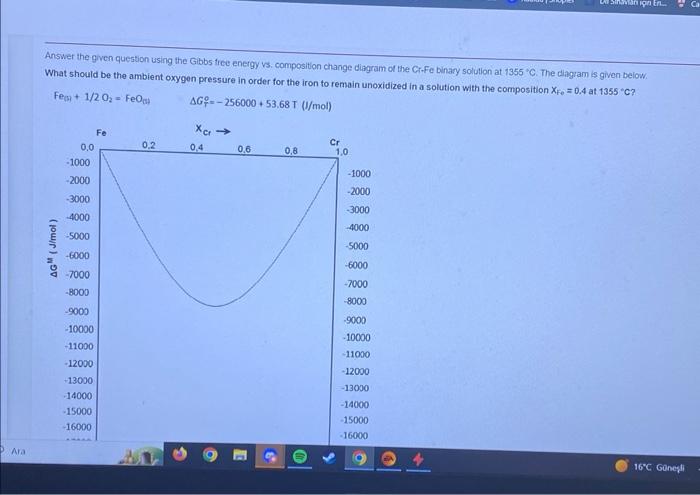
Answer the given question using the Gibbs tree energy vs. composition change diagram of the G.Fe binary solution at 1355C. The cliagram is given below. What should be the ambient oxygen pressure in order for the iron to remain unoxidized in a solution with the composition XFe=0.4 at 13555C ? Fe(B+1/2O2=FeOGHGT0=256000+53.68T(J/mol)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


