Question: Answer the predictions tab In this lab activity, you will explore how stress applied to a variety of systems at equilibrium will affect the direction
Answer the predictions tab 
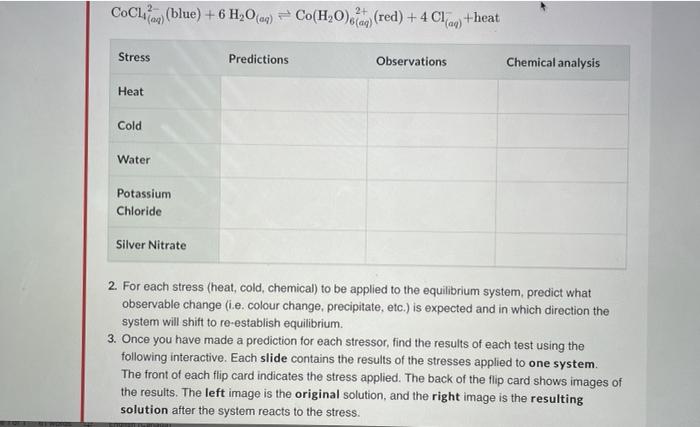
In this lab activity, you will explore how stress applied to a variety of systems at equilibrium will affect the direction in which the system will shift to re-establish equilibrium The following equilibrium systems will be tested in this lab: Cobalt CoCl2(blue) + 6 H20) Co(H20)679 (red) +4 CM +heat Phenolphthalein: HIn(o) (clear) + H200 In wy (pink)+H30) Iron thiocyanate: Fear (pale yellow) + SCN FeSCN, (red) + heat Chromate: 2 CrOla (yellow) + 2 Haq) Cry0% (red)+H30 (ag) Cochon (blue) + 6 H 0(09) = Co(H20) (d) (red) + 4 C1 +heat Stress Predictions Observations Chemical analysis Heat Cold Water Potassium Chloride Silver Nitrate 2. For each stress (heat, cold, chemical) to be applied to the equilibrium system, predict what observable change (i.e. colour change, precipitate, etc.) is expected and in which direction the system will shift to re-establish equilibrium. 3. Once you have made a prediction for each stressor, find the results of each test using the following interactive. Each slide contains the results of the stresses applied to one system. The front of each flip card indicates the stress applied. The back of the flip card shows images of the results. The left image is the original solution, and the right image is the resulting solution after the system reacts to the stress
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


