Question: Answer the three parts to a question. 1a) 1b) For all s electrons in an iron atom (both core and valence), which is the most
Answer the three parts to a question.
1a)
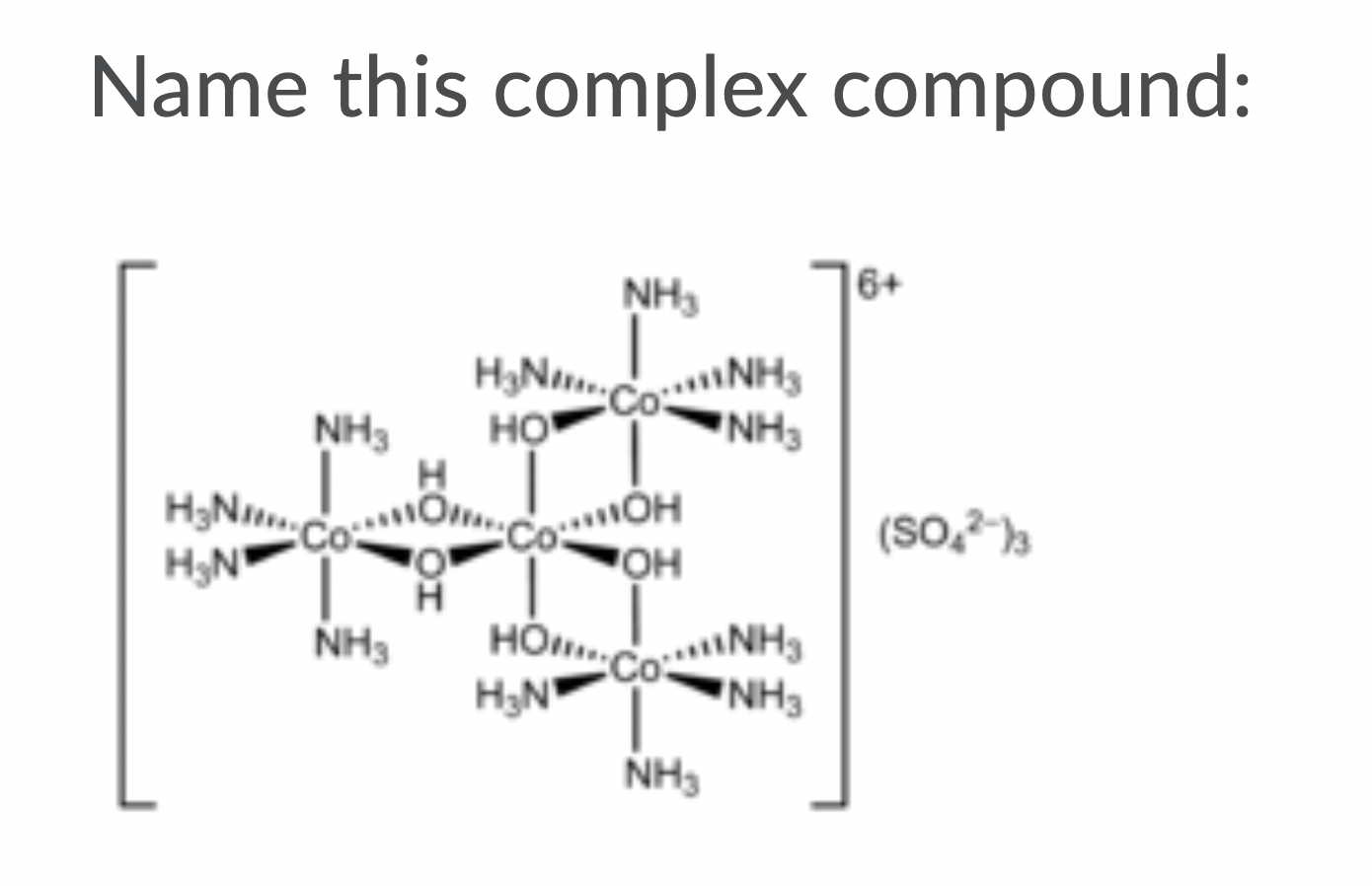
1b)
For all "s" electrons in an iron atom (both core and valence), which is the most tightly held? Which is the most loosely held? Support your answer with a calculation, and show your work.
1c)
Show the molecular orbital diagrams for homonuclear diatoms with and without secondary mixing. Highlight the differences, and discuss how this relates to "hybridization".
Name this complex compound: 6+ NH HNCNH3 NH3 NH3 HO H HNCO.C Co COOH JOH HN NH HO.CONH3 HN NH3 NH3 OH (SO-13
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


