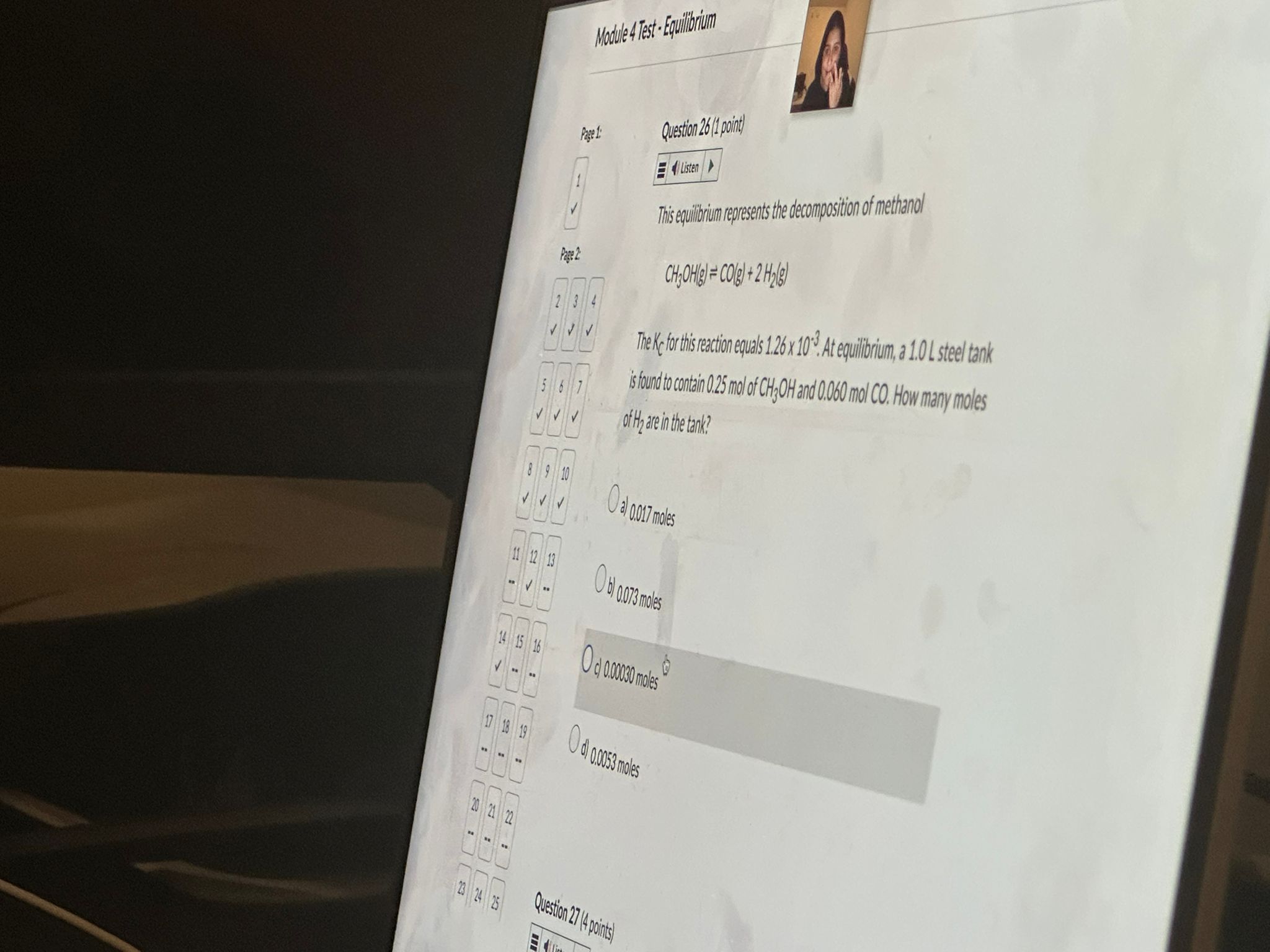
Question: Answer this Module 4 Test . Equilibrium Question 26 (1 point) Page 1 Listen 1 This equilibrium represents the decomposition of methanol Page 2 CH,
Answer this
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


