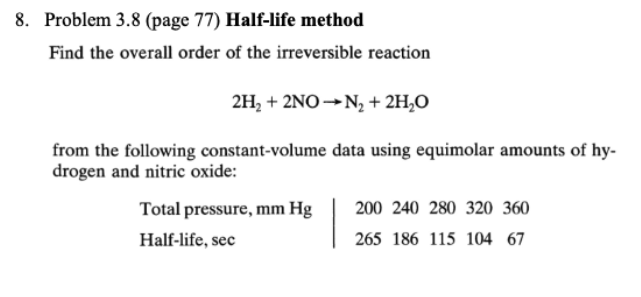
Question: answer this question 8. Problem 3.8 (page 77) Half-life method Find the overall order of the irreversible reaction 2H2 + 2NO -N2 + 2HO from
answer this question
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


