Question: ANSWER THIS QUESTION, PLEASE DONT COPY PASTE YOUR WORK OF ANOTHER PROBLEM JUST BECAUSE IT MENTIONS PROPANE 3. Compressing propane (T. = 369.83 K, P
ANSWER THIS QUESTION, PLEASE DONT COPY PASTE YOUR WORK OF ANOTHER PROBLEM JUST BECAUSE IT MENTIONS PROPANE
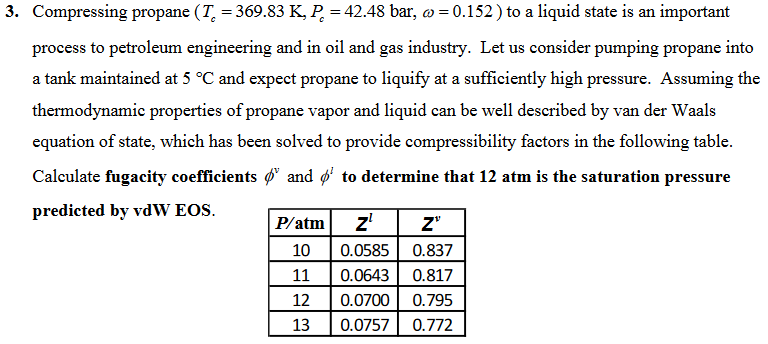
3. Compressing propane (T. = 369.83 K, P = 42.48 bar, w=0.152 ) to a liquid state is an important process to petroleum engineering and in oil and gas industry. Let us consider pumping propane into a tank maintained at 5 C and expect propane to liquify at a sufficiently high pressure. Assuming the thermodynamic properties of propane vapor and liquid can be well described by van der Waals equation of state, which has been solved to provide compressibility factors in the following table. Calculate fugacity coefficients g and $' to determine that 12 atm is the saturation pressure predicted by vdW EOS. P/atm ! z Z" 10 0.0585 0.837 11 0.0643 0.817 12 0.0700 0.795 13 0.0757 0.772
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


