Question: ANSWER THIS QUESTION WITH NUMBERS AND GRAPH. DO NOT USE CHA.TGP.T 4. A final year student was then instructed by his supervisor to elevate the
ANSWER THIS QUESTION WITH NUMBERS AND GRAPH. DO NOT USE CHA.TGP.T
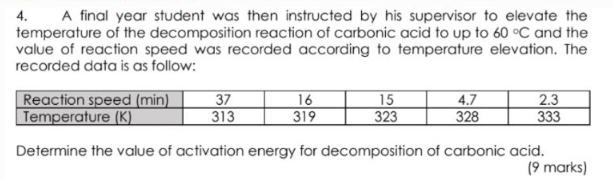
4. A final year student was then instructed by his supervisor to elevate the temperature of the decomposition reaction of carbonic acid to up to 60C and the value of reaction speed was recorded according to temperature elevation. The recorded data is as follow: Determine the value of activation energy for decomposition of carbonic acid. (9 marks) 4. A final year student was then instructed by his supervisor to elevate the temperature of the decomposition reaction of carbonic acid to up to 60C and the value of reaction speed was recorded according to temperature elevation. The recorded data is as follow: Determine the value of activation energy for decomposition of carbonic acid. (9 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


