Question: answer using steps pls Ammonia is synthesised from the catalytic reaction of nitrogen and hydrogen: N2+3H22NH3 For this example, hydrogen is supplied at 50% excess.
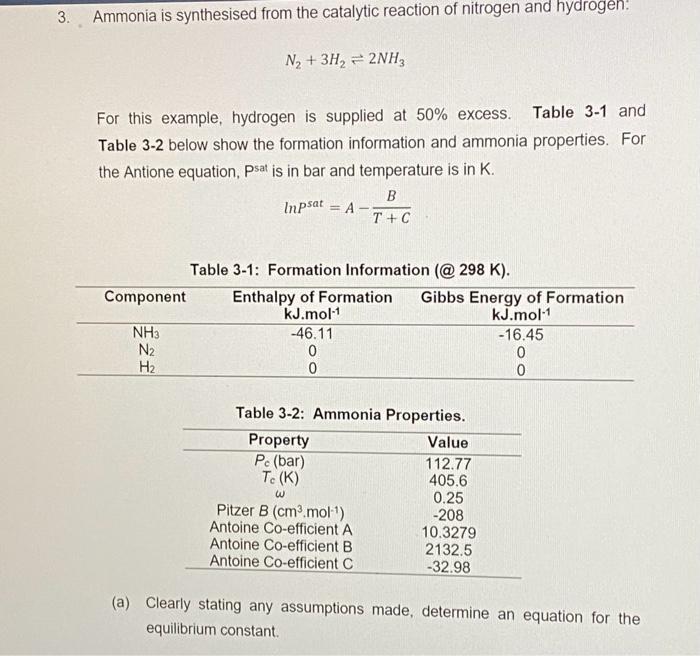

Ammonia is synthesised from the catalytic reaction of nitrogen and hydrogen: N2+3H22NH3 For this example, hydrogen is supplied at 50% excess. Table 31 and Table 3-2 below show the formation information and ammonia properties. For the Antione equation, Psat is in bar and temperature is in K. lnPsat=AT+CB Table 3-1: Formation Information (@298 K). Table 3-2: Ammonia Properties. (a) Clearly stating any assumptions made, determine an equation for the equilibrium constant. (b) It is necessary to optimise the reactor conditions in order to maximize the conversion. Clearly stating any assumptions made, evaluate the conversion at each of the following conditions: (i) 600K and 2 bar (abs). (ii) 600K and one other pressure of your choice. (iii) 2 bar (abs) and one other temperature of your choice. You must clearly explain why you have chosen the pressure and temperature chosen for parts (i) and (ii). (15 marks) (c) Based on your results in part (b), comment on the likely conditions that would optimise the production of ammonia. (2 marks) (d) Assuming the optimised reactor conditions are 500K and 25 bar (abs), determine the Z-factor of ammonia using: (i) Virial equation truncated to two terms. (ii) Soave-Redich-Kwong Equation of State. Comment on your results. (12 marks) Ammonia is synthesised from the catalytic reaction of nitrogen and hydrogen: N2+3H22NH3 For this example, hydrogen is supplied at 50% excess. Table 31 and Table 3-2 below show the formation information and ammonia properties. For the Antione equation, Psat is in bar and temperature is in K. lnPsat=AT+CB Table 3-1: Formation Information (@298 K). Table 3-2: Ammonia Properties. (a) Clearly stating any assumptions made, determine an equation for the equilibrium constant. (b) It is necessary to optimise the reactor conditions in order to maximize the conversion. Clearly stating any assumptions made, evaluate the conversion at each of the following conditions: (i) 600K and 2 bar (abs). (ii) 600K and one other pressure of your choice. (iii) 2 bar (abs) and one other temperature of your choice. You must clearly explain why you have chosen the pressure and temperature chosen for parts (i) and (ii). (15 marks) (c) Based on your results in part (b), comment on the likely conditions that would optimise the production of ammonia. (2 marks) (d) Assuming the optimised reactor conditions are 500K and 25 bar (abs), determine the Z-factor of ammonia using: (i) Virial equation truncated to two terms. (ii) Soave-Redich-Kwong Equation of State. Comment on your results. (12 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


