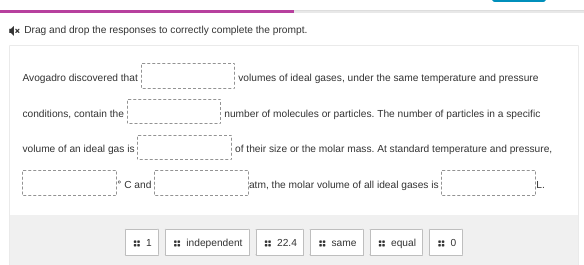
Question: answer (x Drag and drop the responses to correctly complete the prompt. Avogadro discovered that ; volumes of ideal gases, under the same temperature and
answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


