Question: Answers in detail if possible please Method 2: Back titration 0.5307g of the powered aspirin (prepared in Table 1) was taken and added to a
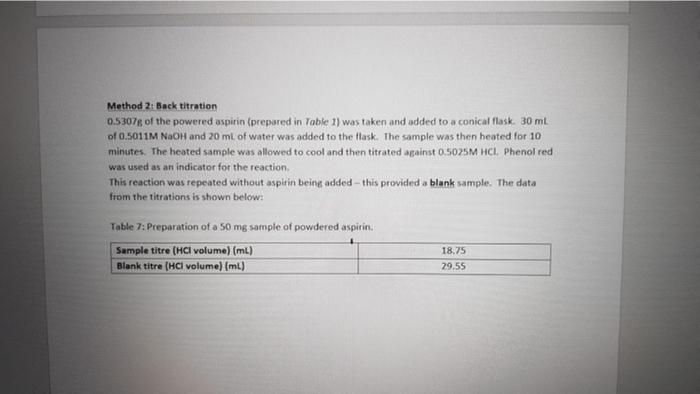

Method 2: Back titration 0.5307g of the powered aspirin (prepared in Table 1) was taken and added to a conical flask. 30 ml of 0.5011M NaOH and 20 ml of water was added to the flask. The sample was then heated for 10 minutes. The heated sample was allowed to cool and then titrated against 0.5025M HCI. Phenol red was used as an indicator for the reaction. This reaction was repeated without aspirin being added this provided a blank sample. The data from the titrations is shown below: Table 7: Preparation of a 50 mg sample of powdered aspirin. Sample titre (HCl volume) (ml) Blank titre (HCl volume) (ml) 18.75 29.55 Back-titration Questions: 8. Why was the sample heated? (2 marks) 9. Why was a blank titration performed? (2 marks) 10. Using the information provided (back-titration assay) work out the % of stated amount for aspirin. (5 marks) 11. Write out a full chemical equation for the reaction carried out in the back-titration. (2 marks) 12. Write out the full reaction mechanism for the reaction carried out in the back- titration. (5 marks) 13. Is the spectrophotometry assay more or less specific than the back-titration assay? Explain your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


