Question: Answers provided. Please show step by step on solving for each part. One method of producing propylene is the dehydrogenation of propane (C3H8) to propylene
Answers provided. Please show step by step on solving for each part.
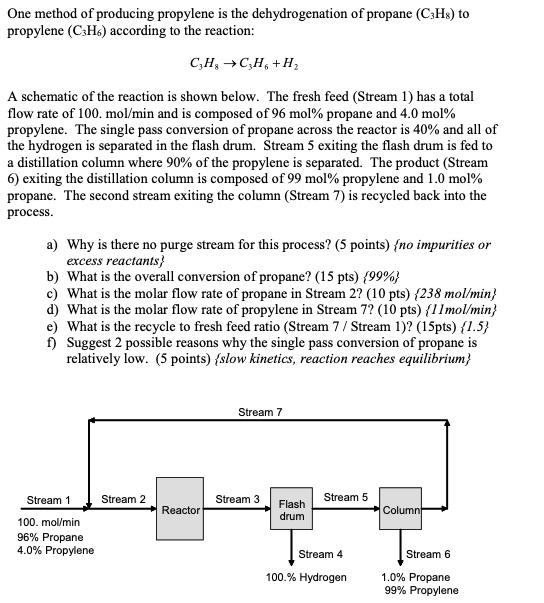
One method of producing propylene is the dehydrogenation of propane (C3H8) to propylene (C3H6) according to the reaction: C3H8C3H6+H2 A schematic of the reaction is shown below. The fresh feed (Stream 1 ) has a total flow rate of 100.mol/min and is composed of 96mol% propane and 4.0mol% propylene. The single pass conversion of propane across the reactor is 40% and all of the hydrogen is separated in the flash drum. Stream 5 exiting the flash drum is fed to a distillation column where 90% of the propylene is separated. The product (Stream 6) exiting the distillation column is composed of 99mol% propylene and 1.0mol% propane. The second stream exiting the column (Stream 7) is recycled back into the process. a) Why is there no purge stream for this process? (5 points) \{no impurities or excess reactants? b) What is the overall conversion of propane? (15 pts) {99%} c) What is the molar flow rate of propane in Stream 2 ? (10 pts) {238mol/min} d) What is the molar flow rate of propylene in Stream 7 ? (10 pts) {11mol/min} e) What is the recycle to fresh feed ratio (Stream 7/ Stream 1) ? (15pts) {1.5} f) Suggest 2 possible reasons why the single pass conversion of propane is relatively low. (5 points) \{slow kinetics, reaction reaches equilibrium\}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


