Question: Any help pls!! STEP BY STEP AND CLEARLY Help plss 1203E Analytical Chemistry (CRN: 20598) Time left 1:28:55 estion 1 yet Calculate the pH of
Any help pls!! STEP BY STEP AND CLEARLY
\Help plss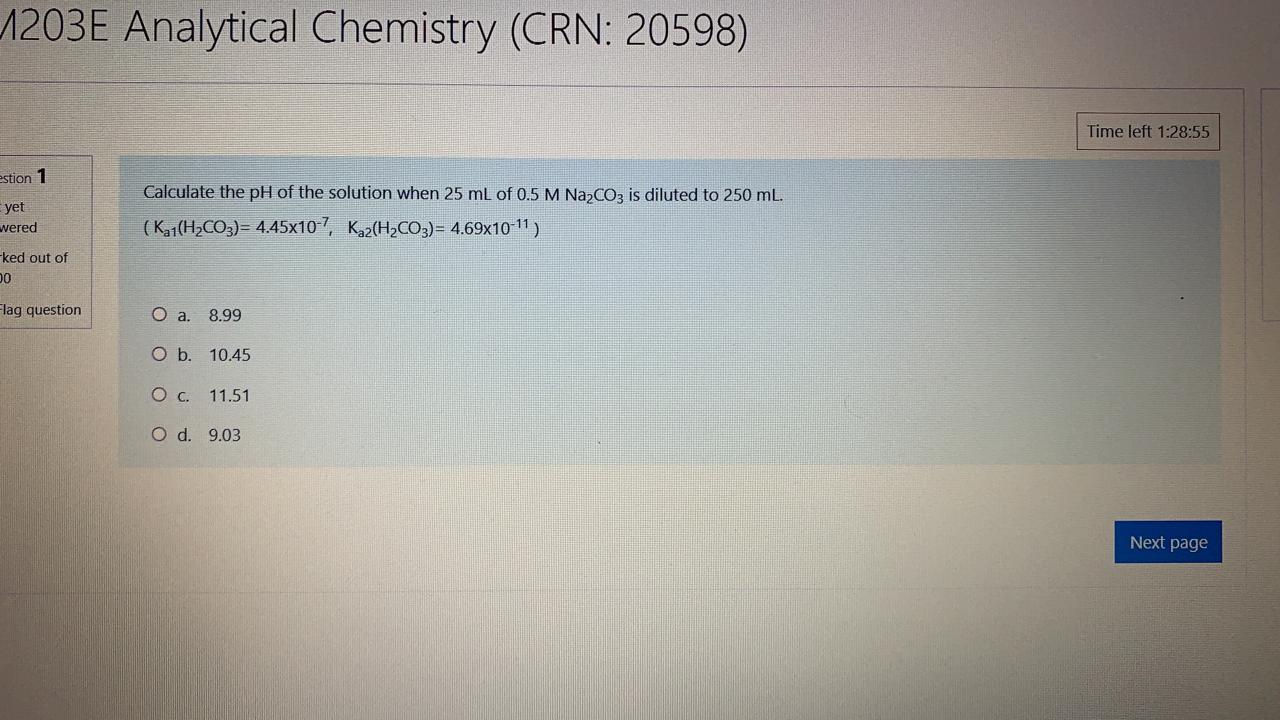
1203E Analytical Chemistry (CRN: 20598) Time left 1:28:55 estion 1 yet Calculate the pH of the solution when 25 mL of 0.5 M Na2CO3 is diluted to 250 ml. (Kg1(H2CO3)= 4.45x10-7, Kaz(H2CO3)= 4.69x10-11) wered -ked out of 0 Flag question O a. 8.99 O b. 10.45 O c. 11.51 O d. 9.03 Next page
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


