Question: anyone can help me answer asap please 1.0 - A = 0.001 0.002 0.9 0.003 Time in minutes mol liter For other Cao change -

anyone can help me answer asap please
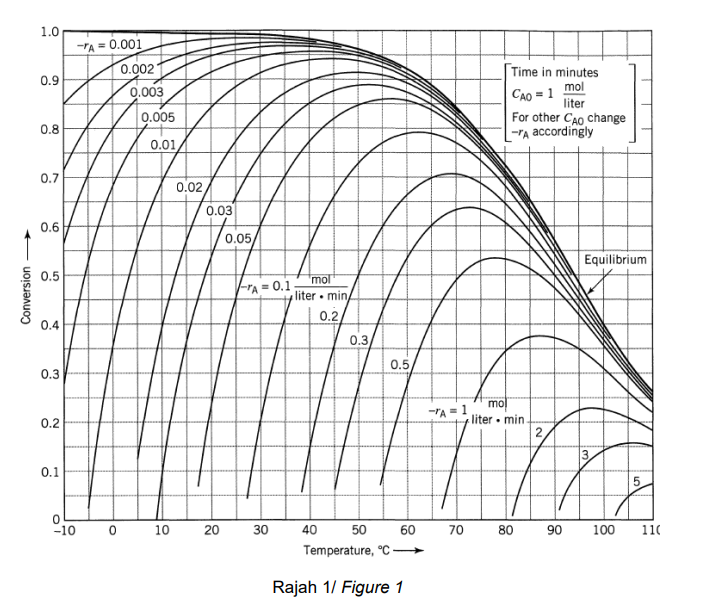
1.0 - A = 0.001 0.002 0.9 0.003 Time in minutes mol liter For other Cao change - accordingly CAO = 1 0.005 0.8 0.01 0.7 0.02 0.03 0.6 0.05 Equilibrium 0.5 Conversion -A=0.1 mol liter. min 0.2/ 0.4 0.3 0.5 0.3 -A=1 mo liter . min 0.2 2 0.1 5 0 -10 0 10 20 30 60 70 80 90 100 110 40 50 Temperature, C Rajah 11 Figure 1 A reversible first order exothermic liquid reaction (AR) to be reacted using optimal progression in plug flow reactor (PER). The plot of conversion against temperature at different reaction rate as shown in Figure 1. The max allowable temperature is 70C and the initial concentration of A is 1 mol/L. a) Calculate the space time (t) and volume for the PFR if 90% conversion of the products to be achieved. The volumetric flowrate is 0.26 m3/min. (6 marks) b) Estimate the temperature outlet of the reaction and final concentration of the reactant A for the reaction in (a) (2 marks) c) You are task to replace the current PFR as it is not functioning well and are damaged. The available replacement is a glass-lined 0.7 m2 mixed flow reactor (MFR) that can only be operated adiabatically. The MFR is to be operated with an inlet temperature of 25C, feed volumetric flow rate of 0.26 m3/min and the inlet concentration of A to be 1 mol/L. Important constraint to be aware of is that the reaction taking place is rather unstable and it cannot exceed the operating temperature of 65C. i) ii) Can you use the available MFR to replace the current damaged PFR, if it will be operated adiabatically? If so, what will be the conversion of A? If you found that you cannot use the glass lined MFR at the current operating condition, suggest one possible modification to the condition in order to enable you to utilize it. AH, = -50kJ/mol; Cp = 0.75 kJ/mol/C (Assume Cp and AH, are independent of temperature)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


