Question: Appendix A data question Due by Thursday The evolution of a closed quantum system is described by a unitary transformation, i.e., the state [(t1)) of
Appendix A data
question Due by Thursday
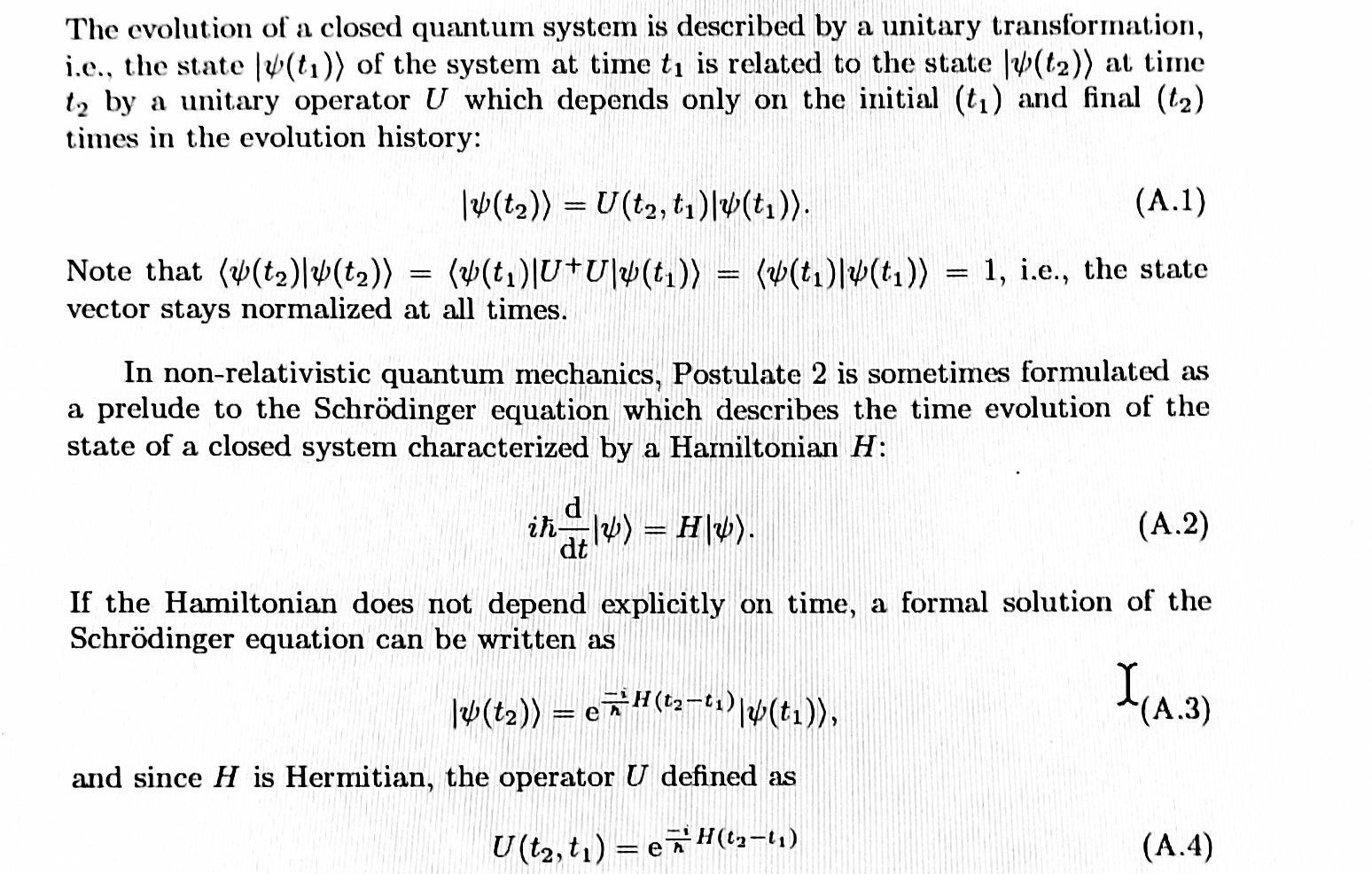
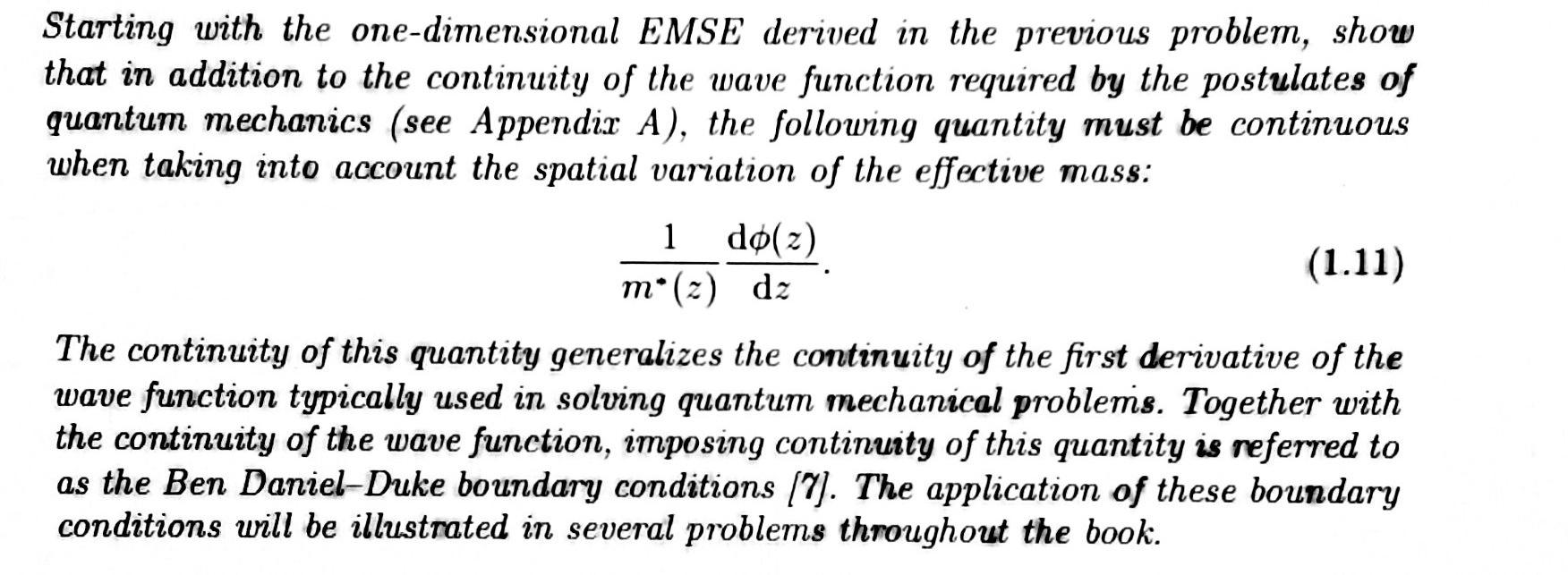
The evolution of a closed quantum system is described by a unitary transformation, i.e., the state [(t1)) of the system at time ty is related to the state [W(t2)) at time t2 by a unitary operator U which depends only on the initial (t) and final (t2) times in the evolution history: 14(t2)) = U(t2, t14(t1)). (A.1) Note that (v(t2)+(t2)) = (v(t.U+U\*(t1)) = (v(t1) 4(t1)) = 1, i.e., the state , vector stays normalized at all times. = In non-relativistic quantum mechanics, Postulate 2 is sometimes formulated as elude to the Schrdinger equation which describes the time evolution of the state of a closed system characterized by a Hamiltonian H: a in a IV) = H\\). (A.2) If the Hamiltonian does not depend explicitly on time, a formal solution of the Schrdinger equation can be written as (t:)) = exH(ta-ti (tv)), I(4.3) e and since H is Hermitian, the operator U defined as U(t2, t) = e 5H(02-11) (A.4) Starting with the one-dimensional EMSE derived in the previous problem, show that in addition to the continuity of the wave function required by the postulates of quantum mechanics (see Appendix A), the following quantity must be continuous when taking into account the spatial variation of the effective mass: 1 m*(z) dz do(z) (1.11) The continuity of this quantity generalizes the continuity of the first derivative of the wave function typically used in solving quantum mechanical problems. Together with the continuity of the wave function, imposing continuity of this quantity is referred to as the Ben Daniel-Duke boundary conditions (7). The application of these boundary conditions will be illustrated in several problems throughout the book
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


