Question: applied reaction engineering A second order, elementary reaction 2AB occurs in a liquid phase CSTR, with the reaction rate constant k=0.14 Lmol1S1. The feed concentration
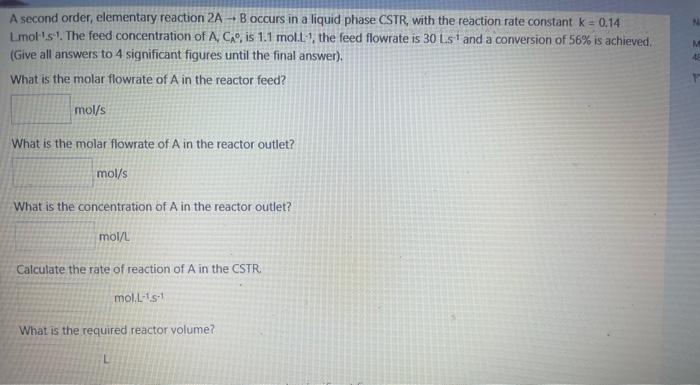
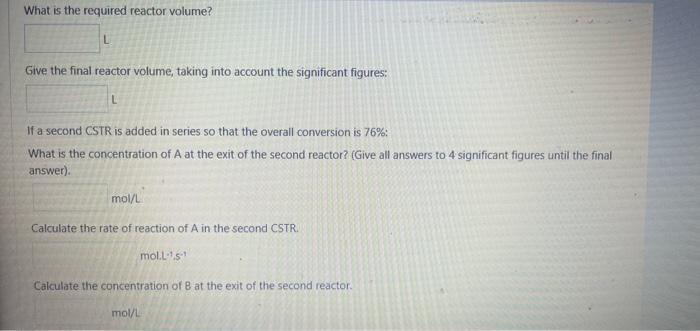

A second order, elementary reaction 2AB occurs in a liquid phase CSTR, with the reaction rate constant k=0.14 Lmol1S1. The feed concentration of A,CA, is 1.1molL1, the feed flowrate is 30LS1 and a conversion of 56% is achieved. (Give all answers to 4 significant figures until the final answer). What is the molar flowrate of A in the reactor feed? What is the molar flowrate of A in the reactor outlet? What is the concentration of A in the reactor outlet? mol/L Calculate the rate of reaction of A in the CSTR. molL1s1 What is the required reactor volume? What is the required reactor volume? Give the final reactor volume, taking into account the significant figures: If a second CSTR is added in series so that the overall conversion is 76% : What is the concentration of A at the exit of the second reactor? (Give all answers to 4 significant figures until the final answer). Calculate the rate of reaction of A in the second CSTR. mol.L-1.m1 Cakculate the concentration of B at the exit of the second reactor. mol/L What is the concentration of A at the exit of the second reactor? (Give all answers to 4 significant figures until the final answer). Calculate the rate of reaction of A in the second CSTR. mol1s1 Calculate the concentration of B at the exit of the second reactor. 10l/L Calculate the reactor volume of the second reactor. Give the final volume for the second reactor, taking into account the significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


