Question: applied reaction engineering For the elementary reaction A+2BC+D Write an expression for the rate of reaction of A. (Use +1 or -1 to indicate a
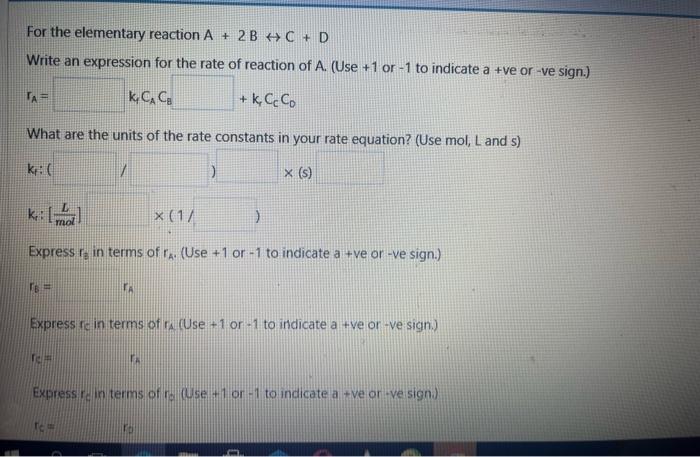
For the elementary reaction A+2BC+D Write an expression for the rate of reaction of A. (Use +1 or -1 to indicate a + ve or-ve sign.) kA=CAC8+kBCCCD What are the units of the rate constants in your rate equation? (Use mol, L and s) Express rg in terms of rA. (Use +1 or -1 to indicate a + ve or -ve sign.) r6= Express fic in terms of ra (Use +1 or -1 to indicate a +ve or -ve sign.) Express re in terms of ro (Use +1 or -1 to indicate a + ve or -ve sign)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


