Question: *** Applying the Energy Balance: Note any table or formula you are using. A closed, rigid tank is filled with water initially at the critical
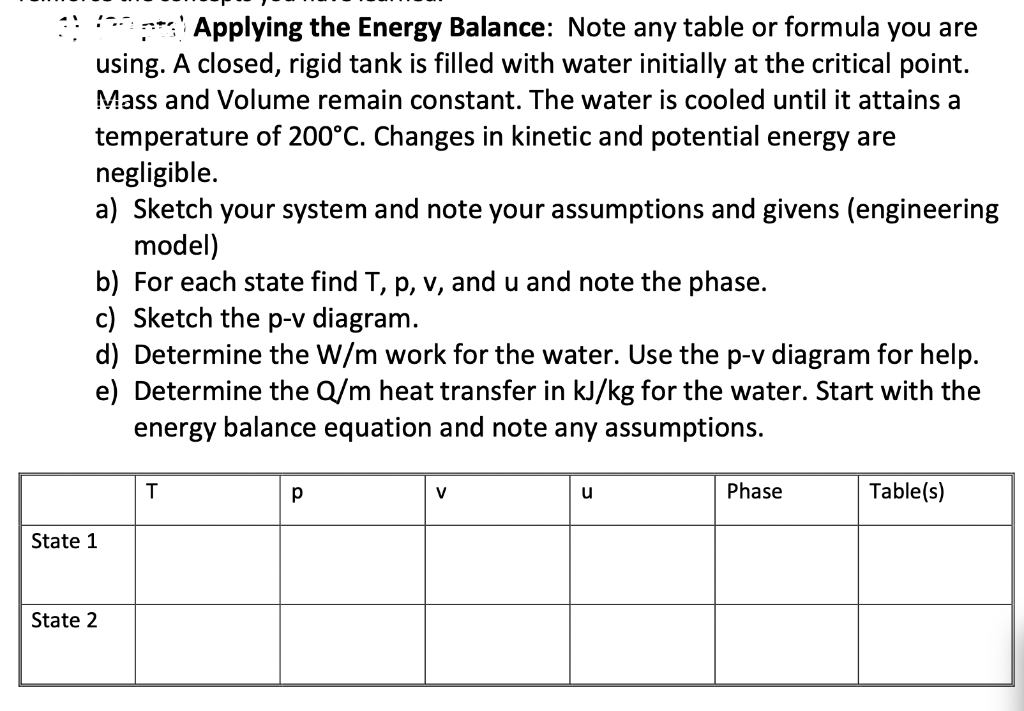
*** Applying the Energy Balance: Note any table or formula you are using. A closed, rigid tank is filled with water initially at the critical point. Mass and Volume remain constant. The water is cooled until it attains a temperature of 200C. Changes in kinetic and potential energy are negligible. a) Sketch your system and note your assumptions and givens (engineering model) b) For each state find T, p, v, and u and note the phase. c) Sketch the p-v diagram. d) Determine the W/m work for the water. Use the p-v diagram for help. e) Determine the Q/m heat transfer in kJ/kg for the water. Start with the energy balance equation and note any assumptions. T V u Phase Table(s) State 1 State 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


