Question: = = ar2 = uw ( 9 ) { Vibrations in the diatomic molecule CO can be approximated as a harmonic oscillator, where the angular
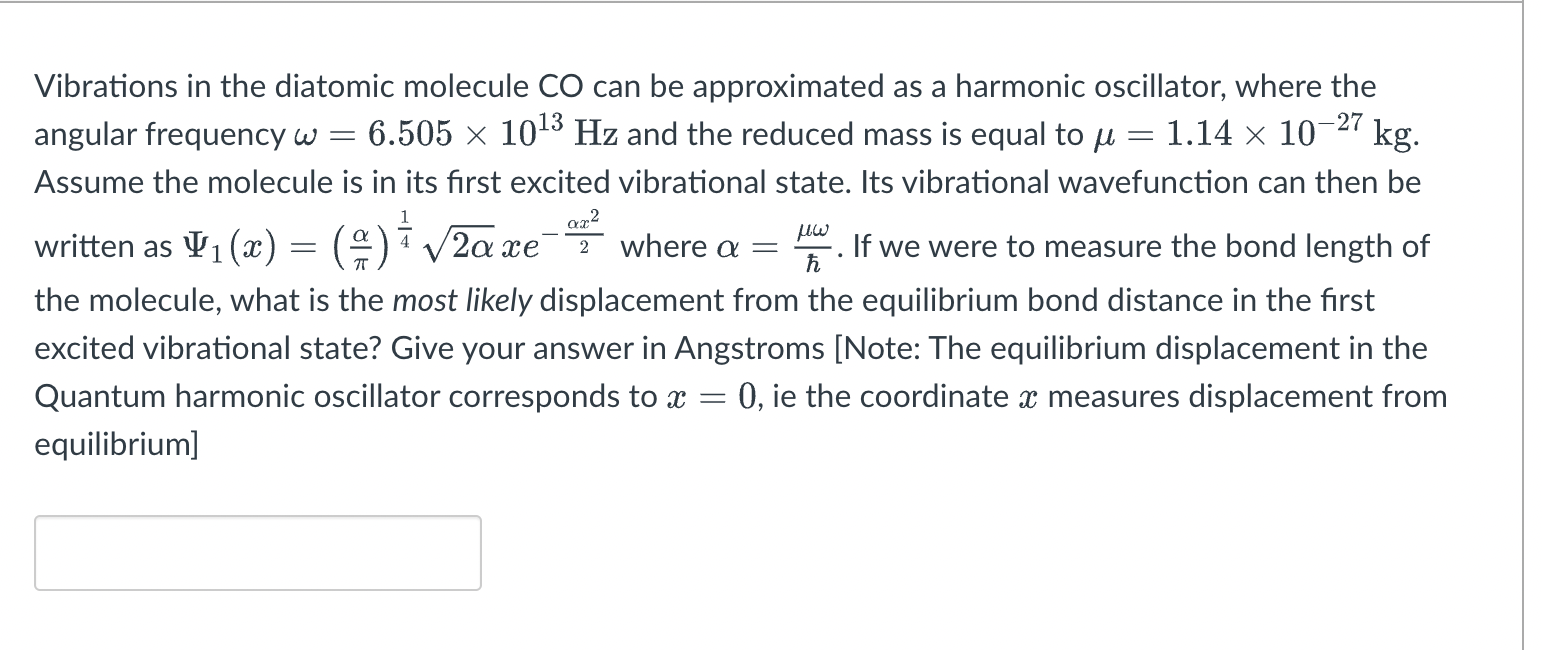
= = ar2 = uw ( 9 ) { Vibrations in the diatomic molecule CO can be approximated as a harmonic oscillator, where the angular frequency w = 6.505 x 1013 Hz and the reduced mass is equal to u 1.14 x 10-27 kg. Assume the molecule is in its first excited vibrational state. Its vibrational wavefunction can then be written as V1(x) = V2ace where a = If we were to measure the bond length of the molecule, what is the most likely displacement from the equilibrium bond distance in the first excited vibrational state? Give your answer in Angstroms [Note: The equilibrium displacement in the Quantum harmonic oscillator corresponds to x = 0, ie the coordinate x measures displacement from equilibrium] 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


