Question: A hypothetical phase diagram is shown in Figure 11-26. (a) Are there any intermetallic compounds present? If so, identify them and determine whether they are
A hypothetical phase diagram is shown in Figure 11-26.
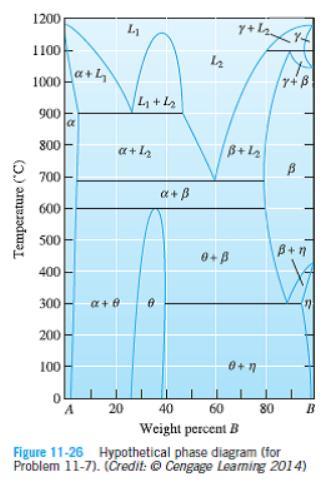
(a) Are there any intermetallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric.
(b) Identify the solid solutions present in the system. Is either material A or B allotropic? Explain.
(c) Identify the three-phase reactions by writing down the temperature, the reaction in equation form, the composition of each phase in the reaction, and the name of the reaction.
1200 L1 1100 1000a+4 r+B L +L2 900 800 a+ l2 B+12 700 a+B 600 500 B+n 400 300 a+ e 200 100 A 20 40 60 80 Weight percent B Figure 11-26 Hypothetical phase diagram (for Problem 11-7). (Credit: Cengage Leaming 2014) Temperature ("C)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


