Question: ART C: Why is a buffer a buffer? 1. Fill out the table above for Part C 2. What is the ratio of A /
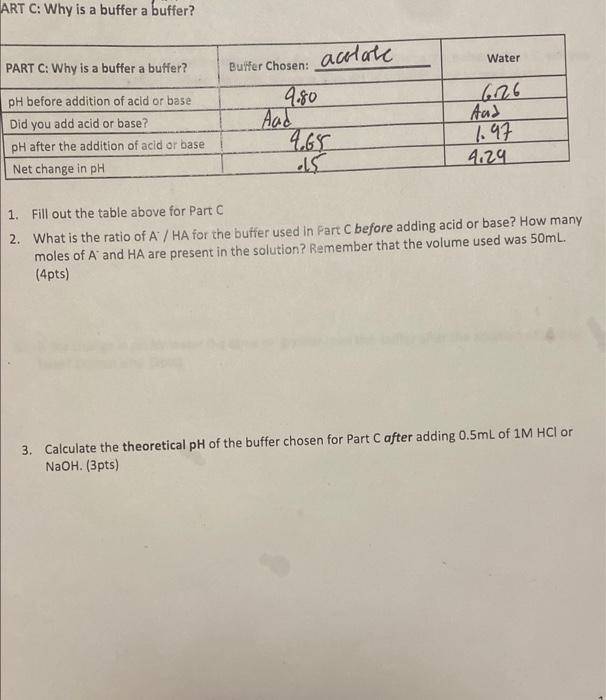
ART C: Why is a buffer a buffer? 1. Fill out the table above for Part C 2. What is the ratio of A / HA for the buffer used in Fart C before adding acid or base? How many moles of Aand HA are present in the solution? Remember that the volume used was 50mL. (4pts) 3. Calculate the theoretical pH of the buffer chosen for Part C after adding 0.5mL of 1MHCl or NaOH. (3pts) ART C: Why is a buffer a buffer? 1. Fill out the table above for Part C 2. What is the ratio of A / HA for the buffer used in Fart C before adding acid or base? How many moles of Aand HA are present in the solution? Remember that the volume used was 50mL. (4pts) 3. Calculate the theoretical pH of the buffer chosen for Part C after adding 0.5mL of 1MHCl or NaOH. (3pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


