Question: As a process engineer you are hired to design a chemical plant to produce 8 0 , 0 0 0 tons per annum ( t
As a process engineer you are hired to design a chemical plant to produce tons per annum ta of ethylbenzene from benzene and ethylene. Benzene assumed pure and Ethylene containing mole fraction of ethylene, ethane feed are available at and Ethane is inert.
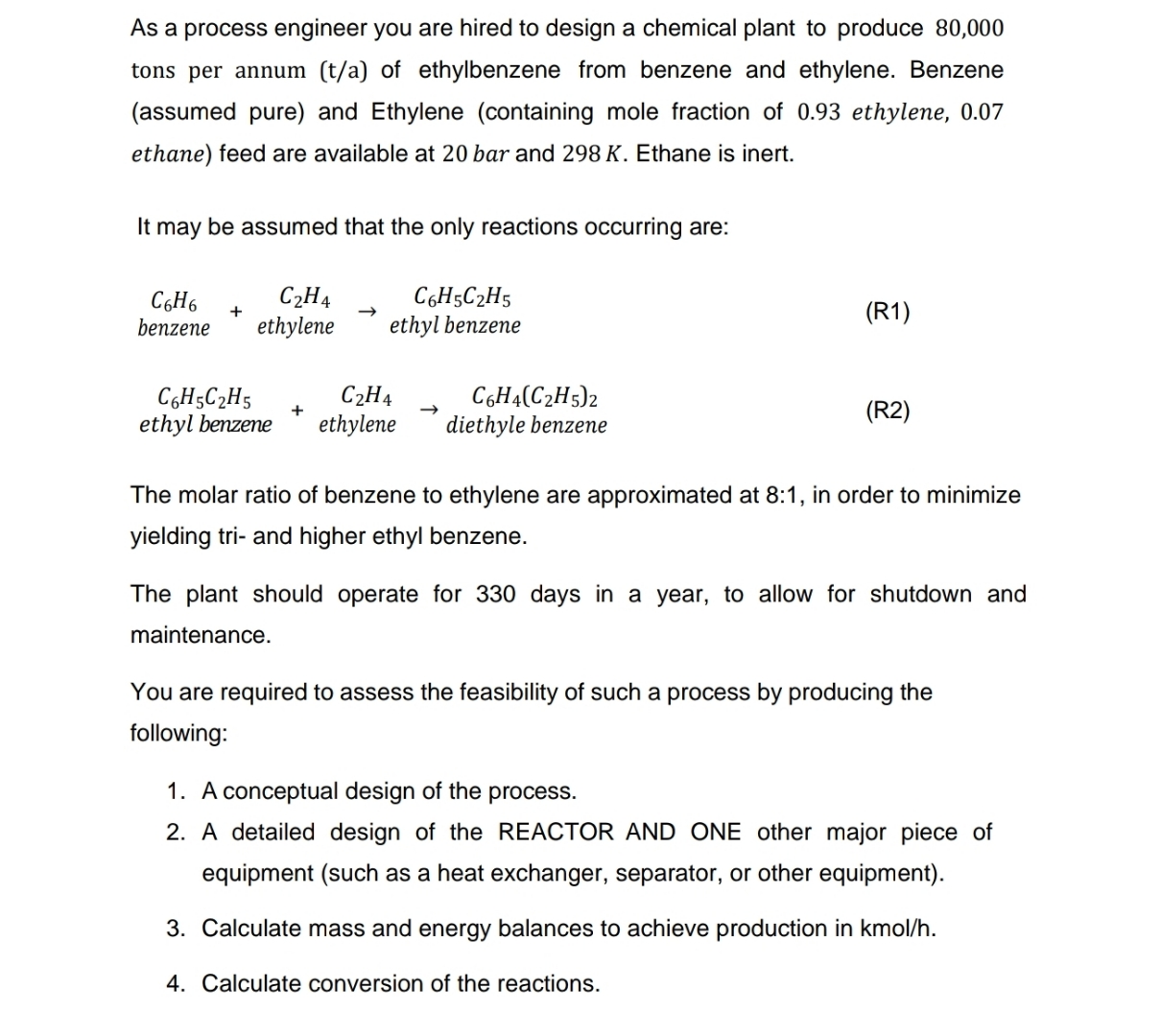
It may be assumed that the only reactions occurring are:
The molar ratio of benzene to ethylene are approximated at : in order to minimize yielding tri and higher ethyl benzene.
The plant should operate for days in a year, to allow for shutdown and maintenance.
You are required to assess the feasibility of such a process by producing the following:
A conceptual design of the process.
A detailed design of the REACTOR AND ONE other major piece of equipment such as a heat exchanger, separator, or other equipment
Calculate mass and energy balances to achieve production in kmo
Calculate conversion of the reactions.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


