Question: Assignment 1 CHEM 233 Calculate the colloidal interaction energy between two colloidal spherical particles of 0.5 micron radius in water at room temperature. Obtain the
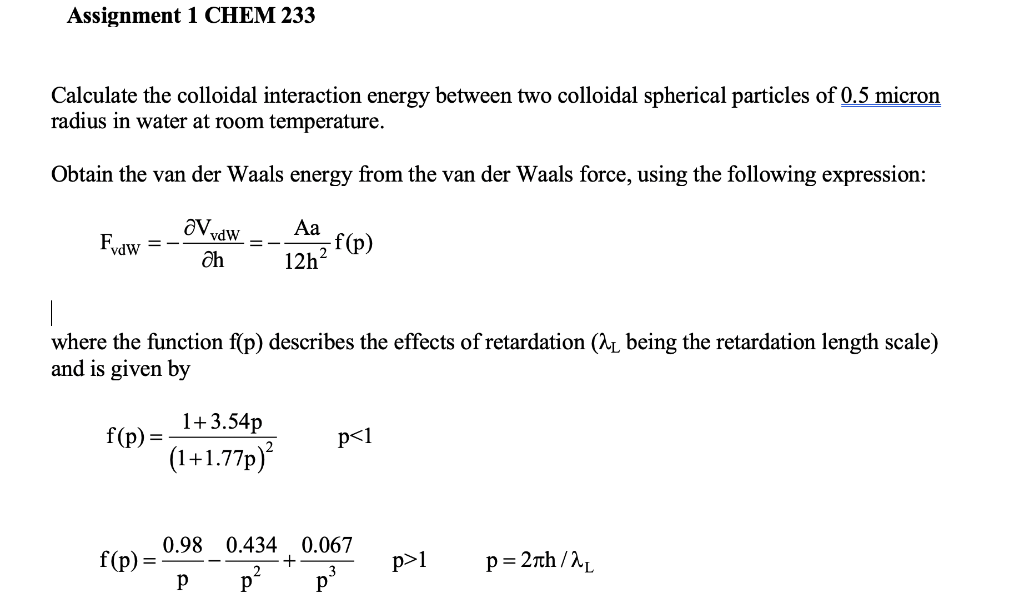
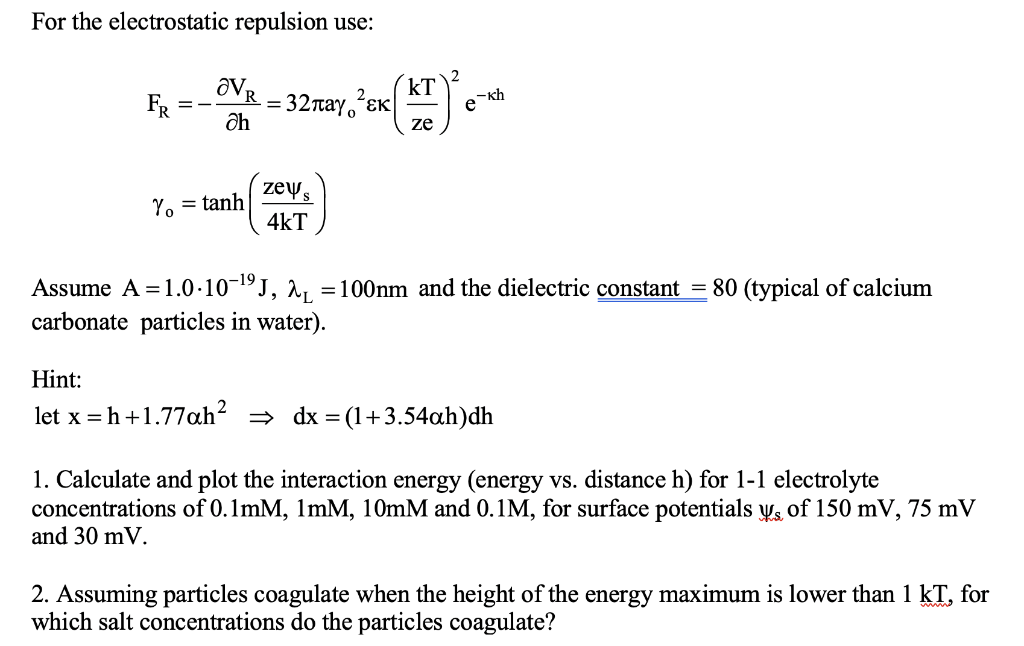
Assignment 1 CHEM 233 Calculate the colloidal interaction energy between two colloidal spherical particles of 0.5 micron radius in water at room temperature. Obtain the van der Waals energy from the van der Waals force, using the following expression: Fyaw ava vdw ah -f(p) 12h where the function f(p) describes the effects of retardation (n being the retardation length scale) and is given by f(p) 1+3.54p (1 +1.77p) p1 p= 27th / For the electrostatic repulsion use: OVR= FR =- k = 32, Oh = () eth ze zeys Ye = tanh) 4kT = Assume A =1.0.10-19, au = 100nm and the dielectric constant = 80 (typical of calcium carbonate particles in water). Hint: let x =h+1.77ah? = dx = (1+3.54ah)dh 1. Calculate and plot the interaction energy (energy vs. distance h) for 1-1 electrolyte concentrations of 0.1mM, 1mM, 10mM and 0.1M, for surface potentials Ys of 150 mV, 75 mV and 30 mV. 2. Assuming particles coagulate when the height of the energy maximum is lower than 1 kT, for which salt concentrations do the particles coagulate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


