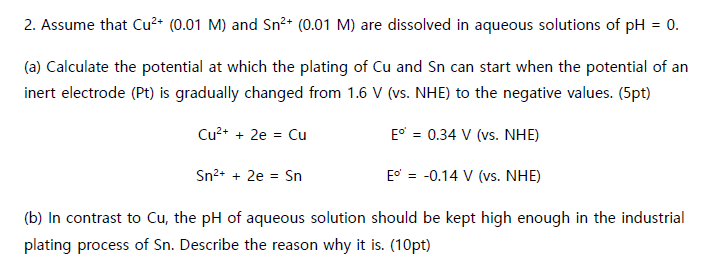
Question: Assume that C u 2 + ( 0 . 0 1 M ) and S n 2 + ( 0 . 0 1 M )
Assume that and are dissolved in aqueous solutions of
a Calculate the potential at which the plating of and can start when the potential of an
inert electrode Pt is gradually changed from vs NHE to the negative values. pt
NHE
NHE
b In contrast to the of aqueous solution should be kept high enough in the industrial
plating process of Describe the reason why it ispt
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


