Question: Assuming equal concentrations and complete dissociation, arrange these aqueous solutions from the lowest (1) to highest boiling (4) points. Co2(SO4)3L2CO3KNO3NiBr3
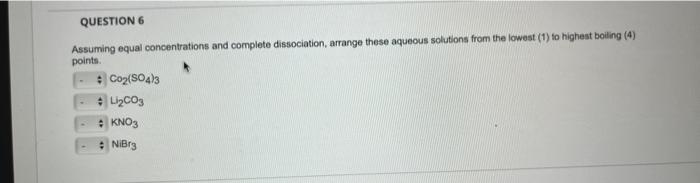
Assuming equal concentrations and complete dissociation, arrange these aqueous solutions from the lowest (1) to highest boiling (4) points. Co2(SO4)3L2CO3KNO3NiBr3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


