Question: Assuming that the unknown diene is responsible for the largest peak on the chromatogram, and that peak areas are proportional to component masses, estimate the
Assuming that the unknown diene is responsible for the largest peak on the chromatogram, and that peak areas are proportional to component masses, estimate the mass of the unknown diene (molecular formula C10H16) in 5.00 g (SS) or 1.00 g (upside down h S) of the eucalyptus oil. Then calculate the mass of maleic anhydride needed to react with that much diene,  and the theoretical yield of the reaction.
and the theoretical yield of the reaction. 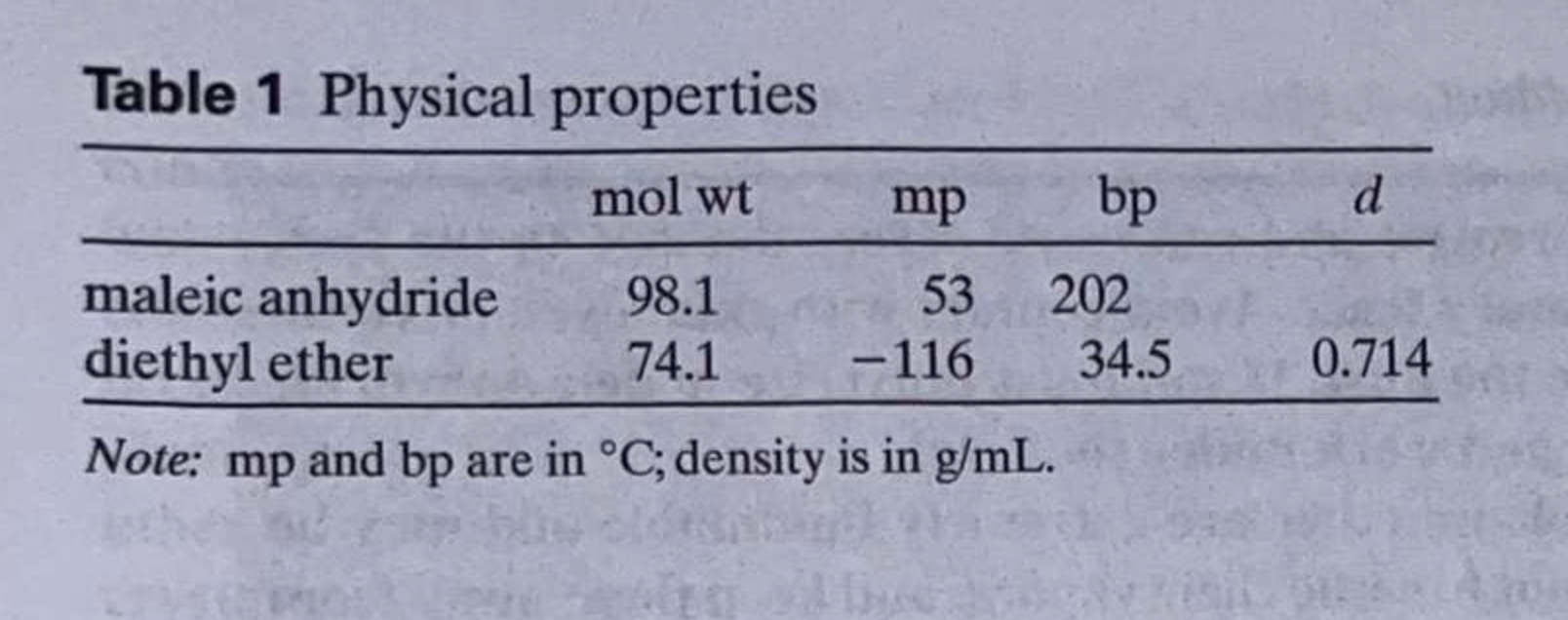
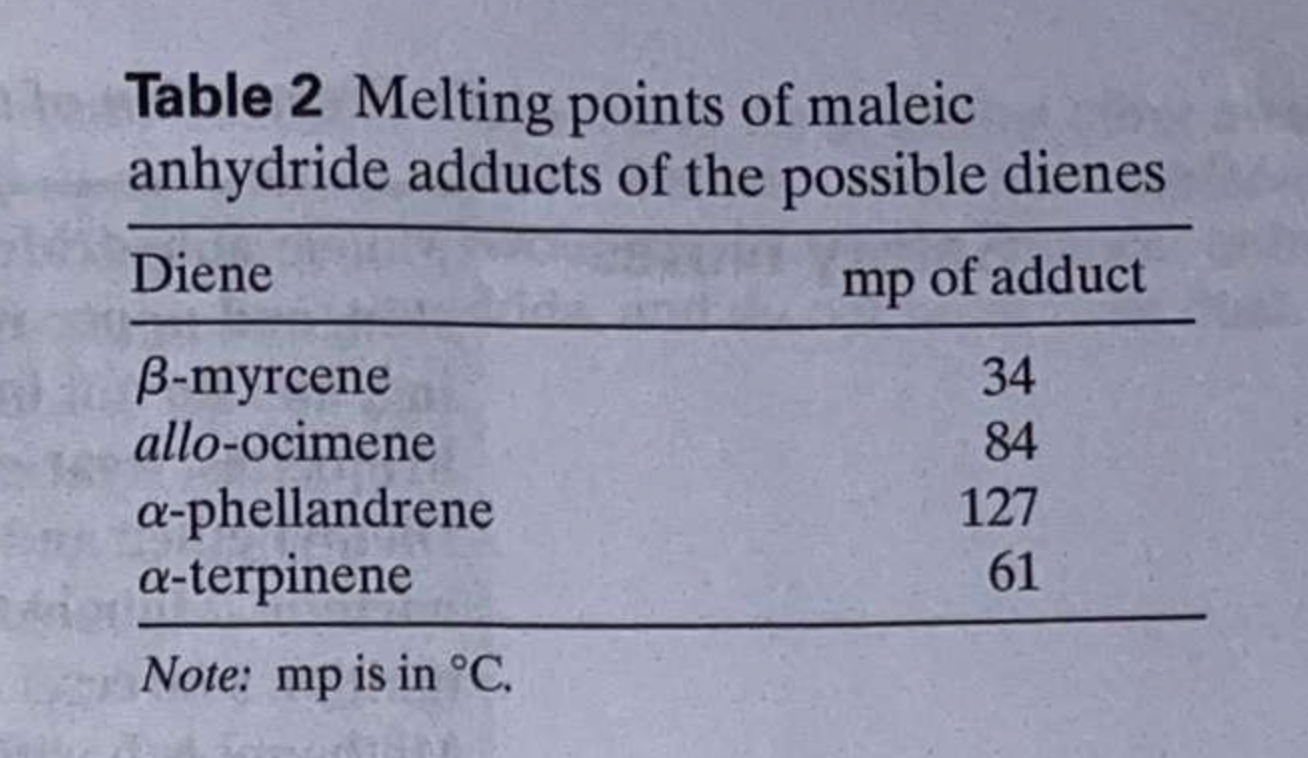
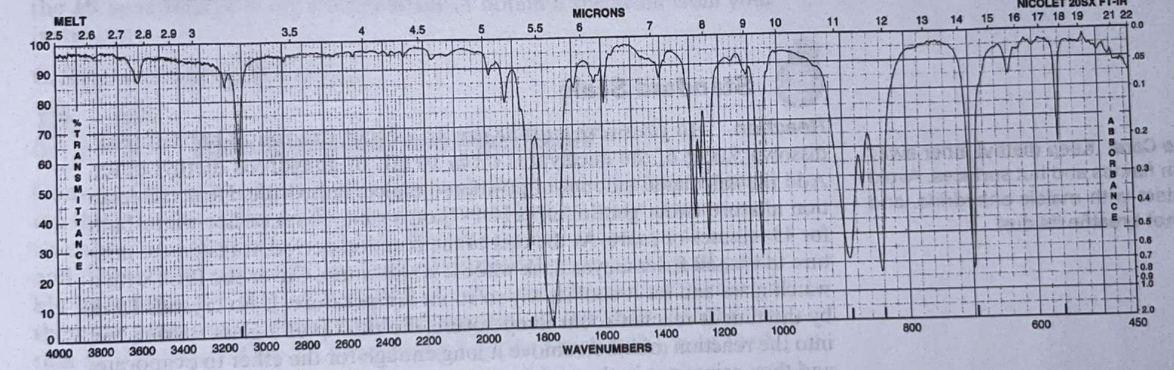
\begin{tabular}{llll} O & 3118.7 & 1240.1 & 840.6 \\ \hline & 1851.1 & 1058.1 & 695.9 \\ 0 & 1778.5 & 890.8 & 561.7 \end{tabular} Numerals of the graph below Table 1 Physical properties Note: mp and bp are in C; density is in g/mL. Table 2 Melting points of maleic anhydride adducts of the possible dienes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


