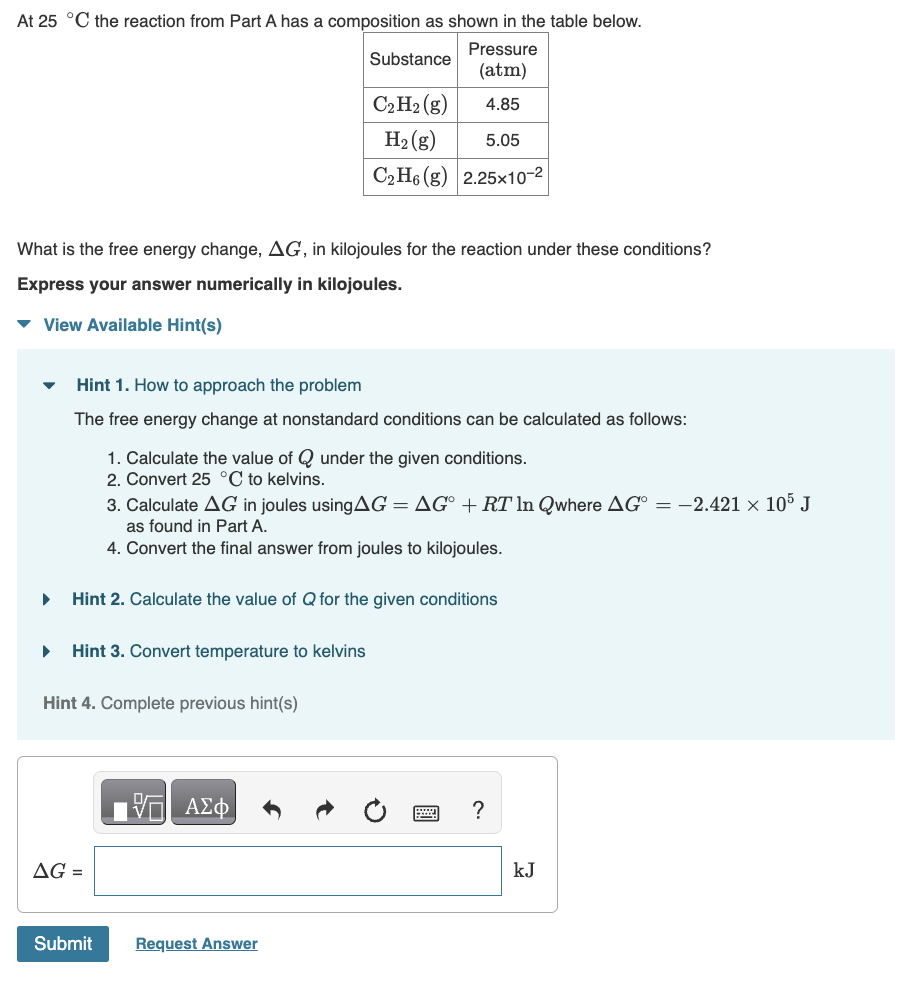
Question: At 2 5 C the reaction from Part A has a composition as shown in the table below. What is the free energy change, G
At the reaction from Part A has a composition as shown in the table below.
What is the free energy change, in kilojoules for the reaction under these conditions?
Express your answer numerically in kilojoules.
View Available Hints
Hint How to approach the problem
The free energy change at nonstandard conditions can be calculated as follows:
Calculate the value of under the given conditions.
Convert to kelvins.
Calculate in joules using where as found in Part A
Convert the final answer from joules to kilojoules.
Hint Calculate the value of for the given conditions
Hint Convert temperature to kelvins
kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


