Question: At 25 C, the equilibrium partial pressures for the reaction 3 A(g) + 4B(g) = 2 C(g) + 3D(g) = were found to be PA
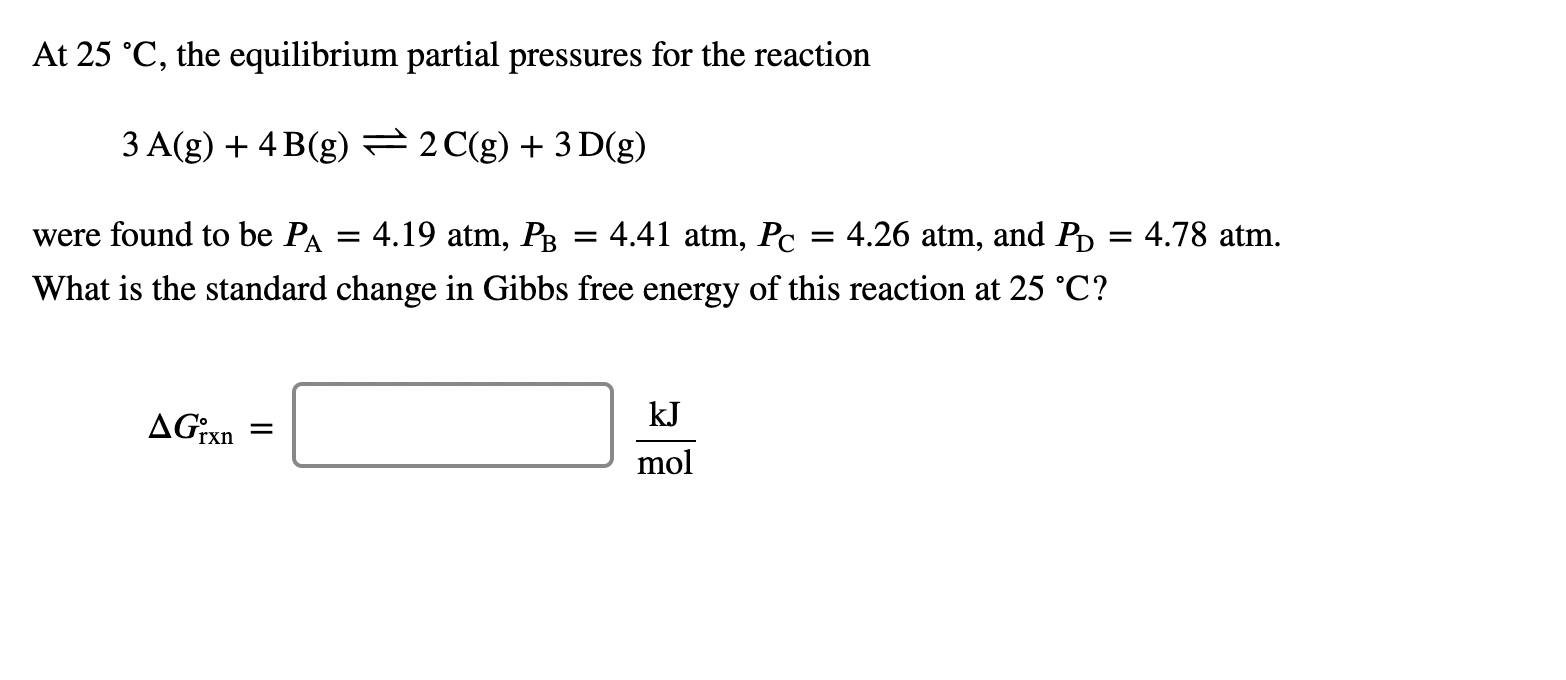
At 25 C, the equilibrium partial pressures for the reaction 3 A(g) + 4B(g) = 2 C(g) + 3D(g) = were found to be PA 4.19 atm, PB 4.41 atm, Pc = 4.26 atm, and Pb = 4.78 atm. What is the standard change in Gibbs free energy of this reaction at 25 C? AGixn = kJ mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


