Question: At 25C, an aqueous solution containing 30.0wt%H2SO4 has a specific gravity of 1.2150. A quantity of the 30.0% solution is needed that contains 495.5kg of

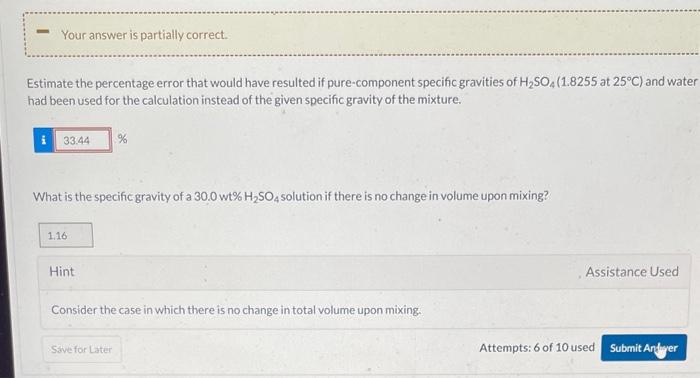
At 25C, an aqueous solution containing 30.0wt%H2SO4 has a specific gravity of 1.2150. A quantity of the 30.0% solution is needed that contains 495.5kg of H2SO4. Required Volume Calculate the required volume (L) of the solution using the given specific gravity. Estimate the percentage error that would have resulted if pure-component specific gravities of H2SO4(1.8255 at 25C) and water had been used for the calculation instead of the given specific gravity of the mixture. What is the specific gravity of a 30.0wt%H2SO4 solution if there is no change in volume upon mixing? Hint Assistance Used Consider the case in which there is no change in total volume upon mixing. Attempts: 6 of 10 used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


