Question: At 27C, five identical rigid 2.0L vessels are filled with N2(g) and sealed. Four of the five vessels also contain a 0.050mol sample of NaHCO3(s),NaBr(s),Cu(s),
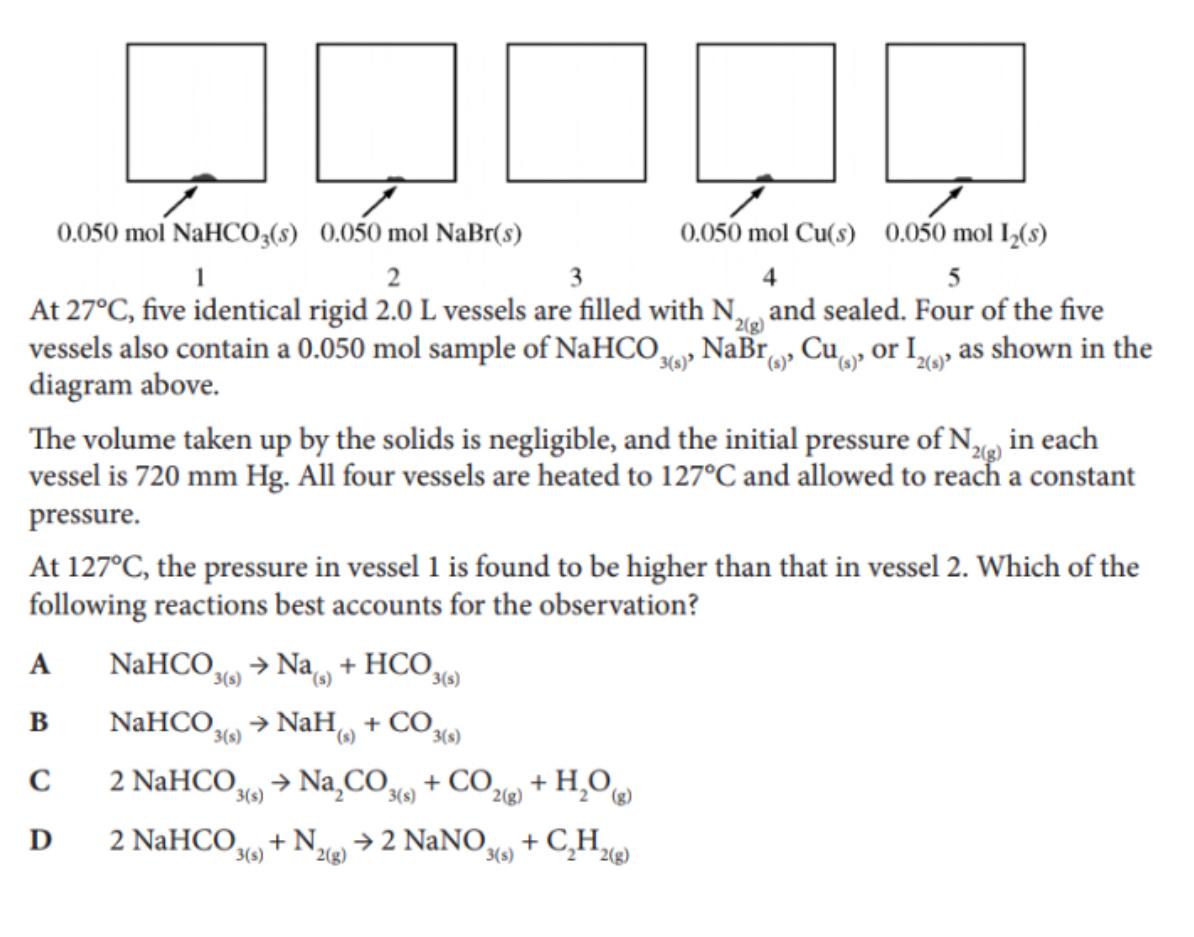
At 27C, five identical rigid 2.0L vessels are filled with N2(g) and sealed. Four of the five vessels also contain a 0.050mol sample of NaHCO3(s),NaBr(s),Cu(s), or I2(s), as shown in the diagram above. The volume taken up by the solids is negligible, and the initial pressure of N2(g) in each vessel is 720mmHg. All four vessels are heated to 127C and allowed to reach a constant pressure. At 127C, the pressure in vessel 1 is found to be higher than that in vessel 2 . Which of the following reactions best accounts for the observation? A NaHCO3(s)Na(s)+HCO3(s) B NaHCO3(s)NaH(s)+CO3(s) C 2NaHCO3(s)Na2CO3(s)+CO2(g)+H2O(g) D 2NaHCO3(s)+N2(g)2NaNO3(s)+C2H2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


