Question: At a certain temperature, a 29.0 - L container holds four gases in equilibrium. Their masses are 3.5gSO3,4.6gSO2,19.6gN and 0.98gN2O What is the value of
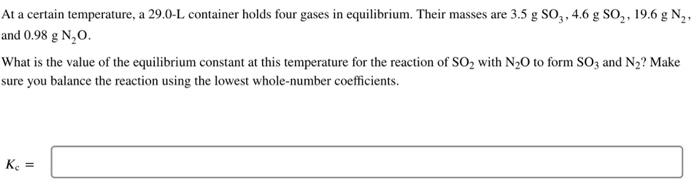
At a certain temperature, a 29.0 - L container holds four gases in equilibrium. Their masses are 3.5gSO3,4.6gSO2,19.6gN and 0.98gN2O What is the value of the equilibrium constant at this temperature for the reaction of SO2 with N2O to form SO3 and N2 ? Make sure you balance the reaction using the lowest whole-number coefficients. Kc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


