Question: At a certain temperature, the equilibrium constant for the chemical reaction shown is 2.54 x 10-3. At equilibrium, the concentration of AB is 2.325
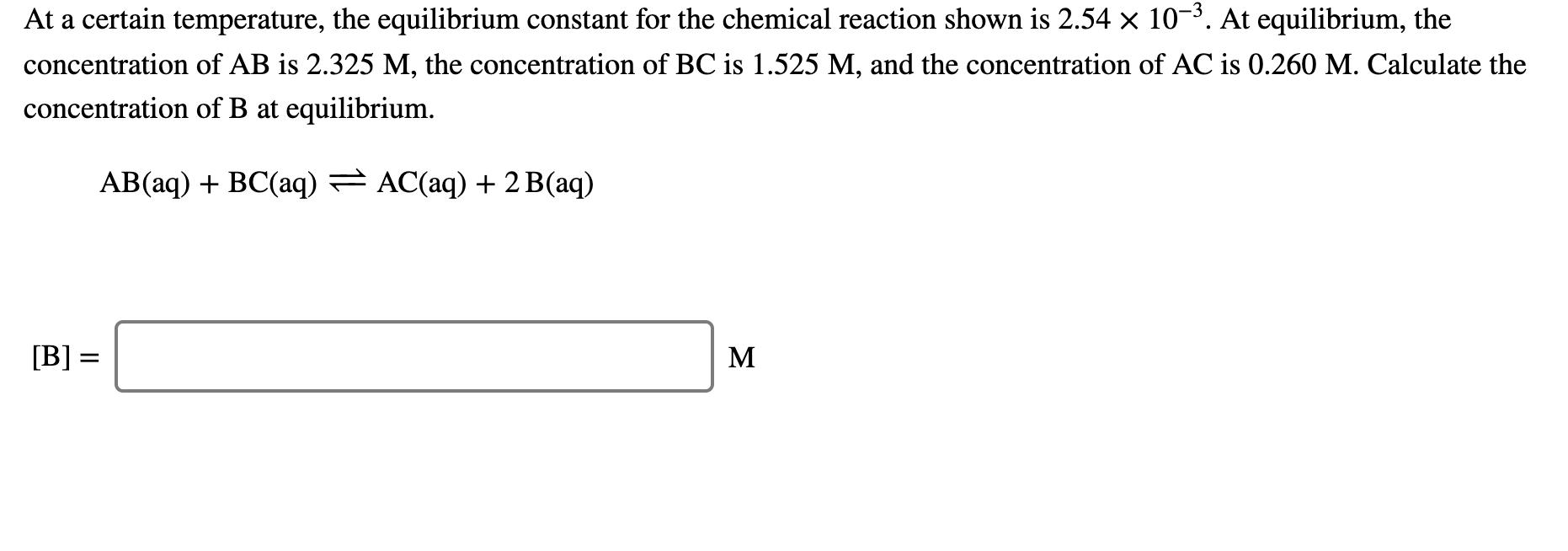
At a certain temperature, the equilibrium constant for the chemical reaction shown is 2.54 x 10-3. At equilibrium, the concentration of AB is 2.325 M, the concentration of BC is 1.525 M, and the concentration of AC is 0.260 M. Calculate the concentration of B at equilibrium. AB(aq) + BC(aq) AC(aq) + 2 B(aq) [B] = M
Step by Step Solution
3.37 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


