Question: At a certain temperature, the equilibrium constant, Kc, for this reaction is 53.3. H2(g)+I2(g)2HI(g)Kc=53.3 At this temperature, 0.300molH2 and 0.300mol2 were placed in a 1.00L
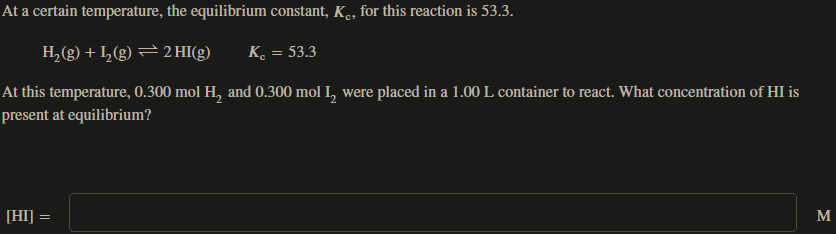
At a certain temperature, the equilibrium constant, Kc, for this reaction is 53.3. H2(g)+I2(g)2HI(g)Kc=53.3 At this temperature, 0.300molH2 and 0.300mol2 were placed in a 1.00L container to react. What concentration of HI is oresent at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


