Question: At a certain temperature the following data were collected for the reaction 21C1 + H2 12 + 2HCI. Initial Concentrations (mol L-1) Initial Rate of
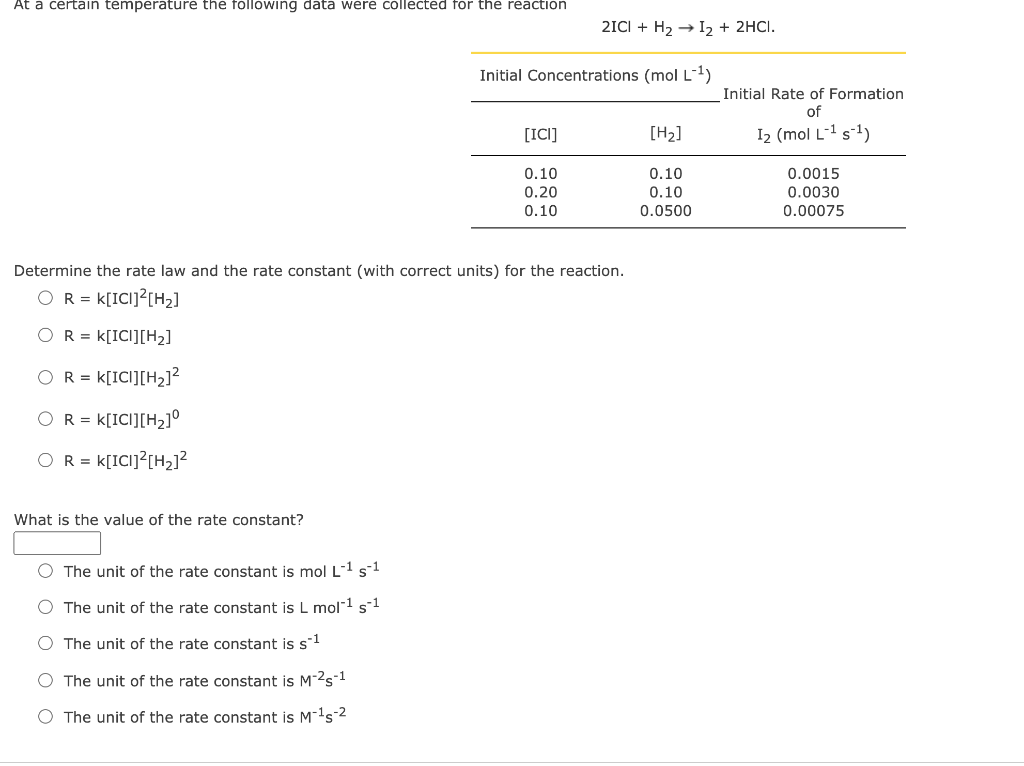
At a certain temperature the following data were collected for the reaction 21C1 + H2 12 + 2HCI. Initial Concentrations (mol L-1) Initial Rate of Formation of 12 (mol (151) [ICI] [H2] 0.10 0.20 0.10 0.10 0.10 0.0500 0.0015 0.0030 0.00075 Determine the rate law and the rate constant (with correct units) for the reaction. O R = K[ICI]2[Hz] O R = K[ICI][H2] O R = K[ICI][H2]2 O R = k[IC][Hz] OR = k[ICI]?[Hz]2 What is the value of the rate constant? O The unit of the rate constant is mol L 1 s 1 O The unit of the rate constant is L mol-1 s-1 The unit of the rate constant is s-1 The unit of the rate constant is M-25-1 The unit of the rate constant is M-15-2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


