Question: At a certain temperature this reaction follows second-order kinetics with a rate constant of 75M1s1 : H2CO3(aq)H2O(aq)+CO2(aq) Suppose a vessel contains H2CO3 at a concentration
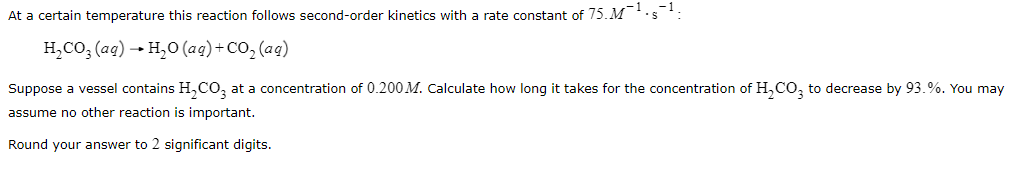
At a certain temperature this reaction follows second-order kinetics with a rate constant of 75M1s1 : H2CO3(aq)H2O(aq)+CO2(aq) Suppose a vessel contains H2CO3 at a concentration of 0.200M. Calculate how long it takes for the concentration of H2CO3 to decrease by 93 . \%. You may assume no other reaction is important. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


