Question: At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g)4NO2(g)+O2(g) When the rate of formation of O2 is 7.3104M/s, the rate of decomposition
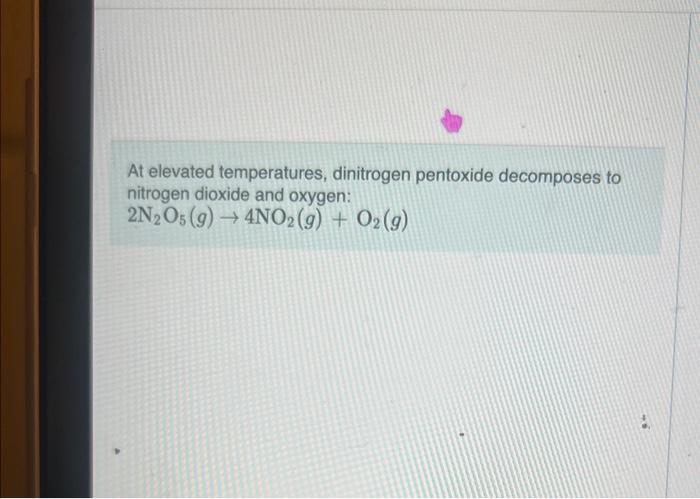
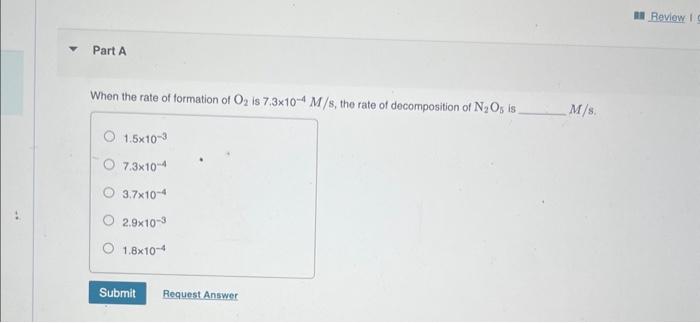
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g)4NO2(g)+O2(g) When the rate of formation of O2 is 7.3104M/s, the rate of decomposition of N2O5 is M/s. 1.51037.31043.71042.91031.8104
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


