Question: At time 1-0, the wavefunction for Hydrogen atom is v (r.0)=(200* (20/1.00 +2.00 + 2/211+31-1) where the subscripts are values of the quantum numbers
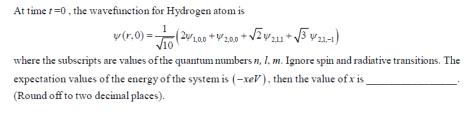
At time 1-0, the wavefunction for Hydrogen atom is v (r.0)=(200* (20/1.00 +2.00 + 2/211+31-1) where the subscripts are values of the quantum numbers n, 1. m. Ignore spin and radiative transitions. The expectation values of the energy of the system is (-xeV), then the value of x is (Round off to two decimal places).
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


